Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity
- PMID: 26507227
- PMCID: PMC4626850
- DOI: 10.1128/mBio.01024-15
Inactivated Influenza Vaccine That Provides Rapid, Innate-Immune-System-Mediated Protection and Subsequent Long-Term Adaptive Immunity
Abstract
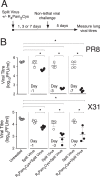
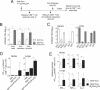
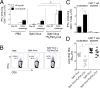
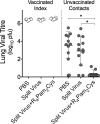
The continual threat to global health posed by influenza has led to increased efforts to improve the effectiveness of influenza vaccines for use in epidemics and pandemics. We show in this study that formulation of a low dose of inactivated detergent-split influenza vaccine with a Toll-like receptor 2 (TLR2) agonist-based lipopeptide adjuvant (R4Pam2Cys) provides (i) immediate, antigen-independent immunity mediated by the innate immune system and (ii) significant enhancement of antigen-dependent immunity which exhibits an increased breadth of effector function. Intranasal administration of mice with vaccine formulated with R4Pam2Cys but not vaccine alone provides protection against both homologous and serologically distinct (heterologous) viral strains within a day of administration. Vaccination in the presence of R4Pam2Cys subsequently also induces high levels of systemic IgM, IgG1, and IgG2b antibodies and pulmonary IgA antibodies that inhibit hemagglutination (HA) and neuraminidase (NA) activities of homologous but not heterologous virus. Improved primary virus nucleoprotein (NP)-specific CD8(+) T cell responses are also induced by the use of R4Pam2Cys and are associated with robust recall responses to provide heterologous protection. These protective effects are demonstrated in wild-type and antibody-deficient animals but not in those depleted of CD8(+) T cells. Using a contact-dependent virus transmission model, we also found that heterologous virus transmission from vaccinated mice to naive mice is significantly reduced. These results demonstrate the potential of adding a TLR2 agonist to an existing seasonal influenza vaccine to improve its utility by inducing immediate short-term nonspecific antiviral protection and also antigen-specific responses to provide homologous and heterologous immunity.
Importance: The innate and adaptive immune systems differ in mechanisms, specificities, and times at which they take effect. The innate immune system responds within hours of exposure to infectious agents, while adaptive immunity takes several days to become effective. Here we show, by using a simple lipopeptide-based TLR2 agonist, that an influenza detergent-split vaccine can be made to simultaneously stimulate and amplify both systems to provide immediate antiviral protection while giving the adaptive immune system time to implement long-term immunity. Both types of immunity induced by this approach protect against vaccine-matched as well as unrelated virus strains and potentially even against strains yet to be encountered. Conferring dual functionality to influenza vaccines is beneficial for improving community protection, particularly during periods between the onset of an outbreak and the time when a vaccine becomes available or in scenarios in which mass vaccination with a strain to which the population is immunologically naive is imperative.
Copyright © 2015 Chua et al.
Figures



Similar articles
-
Broadly Protective CD8+ T Cell Immunity to Highly Conserved Epitopes Elicited by Heat Shock Protein gp96-Adjuvanted Influenza Monovalent Split Vaccine.J Virol. 2021 May 24;95(12):e00507-21. doi: 10.1128/JVI.00507-21. Print 2021 May 24. J Virol. 2021. PMID: 33827939 Free PMC article.
-
Synthetic Toll-like receptor 4 (TLR4) and TLR7 ligands as influenza virus vaccine adjuvants induce rapid, sustained, and broadly protective responses.J Virol. 2015 Mar;89(6):3221-35. doi: 10.1128/JVI.03337-14. Epub 2015 Jan 7. J Virol. 2015. PMID: 25568203 Free PMC article.
-
Bacterium-like particles supplemented with inactivated influenza antigen induce cross-protective influenza-specific antibody responses through intranasal administration.Vaccine. 2012 Jul 6;30(32):4884-91. doi: 10.1016/j.vaccine.2012.04.032. Epub 2012 Apr 23. Vaccine. 2012. PMID: 22537989
-
Intranasal Inactivated Influenza Vaccines: a Reasonable Approach to Improve the Efficacy of Influenza Vaccine?Jpn J Infect Dis. 2016;69(3):165-79. doi: 10.7883/yoken.JJID.2015.560. Jpn J Infect Dis. 2016. PMID: 27212584 Review.
-
Recalling the Future: Immunological Memory Toward Unpredictable Influenza Viruses.Front Immunol. 2019 Jul 2;10:1400. doi: 10.3389/fimmu.2019.01400. eCollection 2019. Front Immunol. 2019. PMID: 31312199 Free PMC article. Review.
Cited by
-
Tetrasubstituted imidazoles as incognito Toll-like receptor 8 a(nta)gonists.Nat Commun. 2021 Jul 16;12(1):4351. doi: 10.1038/s41467-021-24536-4. Nat Commun. 2021. PMID: 34272380 Free PMC article.
-
Prophylactic intranasal administration of a TLR2/6 agonist reduces upper respiratory tract viral shedding in a SARS-CoV-2 challenge ferret model.EBioMedicine. 2021 Jan;63:103153. doi: 10.1016/j.ebiom.2020.103153. Epub 2020 Dec 3. EBioMedicine. 2021. PMID: 33279857 Free PMC article.
-
Bacteria Are a Major Determinant of Orsay Virus Transmission and Infection in Caenorhabditis elegans.bioRxiv [Preprint]. 2024 Mar 18:2023.09.05.556377. doi: 10.1101/2023.09.05.556377. bioRxiv. 2024. Update in: Elife. 2024 Jul 11;12:RP92534. doi: 10.7554/eLife.92534. PMID: 37732241 Free PMC article. Updated. Preprint.
-
Induction of Antiviral Immune Response through Recognition of the Repeating Subunit Pattern of Viral Capsids Is Toll-Like Receptor 2 Dependent.mBio. 2017 Nov 14;8(6):e01356-17. doi: 10.1128/mBio.01356-17. mBio. 2017. Retraction in: mBio. 2024 Aug 14;15(8):e0043024. doi: 10.1128/mbio.00430-24. PMID: 29138299 Free PMC article. Retracted.
-
Bacteria are a major determinant of Orsay virus transmission and infection in Caenorhabditis elegans.Elife. 2024 Jul 11;12:RP92534. doi: 10.7554/eLife.92534. Elife. 2024. PMID: 38990923 Free PMC article.
References
-
- WHO 2009, posting date Influenza (seasonal) fact sheet no. 211. World Health Organization, Geneva, Switzerland: www.who.int/mediacentre/factsheets/fs211/en/index.html.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
