The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer
- PMID: 26503475
- PMCID: PMC4808044
- DOI: 10.18632/oncotarget.5834
The HMG-CoA reductase inhibitor, simvastatin, exhibits anti-metastatic and anti-tumorigenic effects in ovarian cancer
Abstract
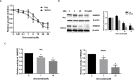
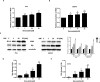
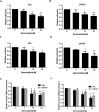
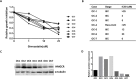
Ovarian cancer is the 5th leading cause of cancer death among women in the United States. The mevalonate pathway is thought to be a potential oncogenic pathway in the pathogenesis of ovarian cancer. Simvastatin, a 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) inhibitor, is a widely used drug for inhibiting the synthesis of cholesterol and may also have anti-tumorigenic activity. Our goal was to evaluate the effects of simvastatin on ovarian cancer cell lines, primary cultures of ovarian cancer cells and in an orthotopic ovarian cancer mouse model. Simvastatin significantly inhibited cellular proliferation, induced cell cycle G1 arrest and apoptosis, and caused cellular stress via reduction in the enzymatic activity of HMGCR and inhibition of the MAPK and mTOR pathways in ovarian cancer cells. Furthermore, simvastatin induced DNA damage and reduced cell adhesion and invasion. Simvastatin also exerted anti-proliferative effects on primary cell cultures of ovarian cancer. Treatment with simvastatin in an orthotopic mouse model reduced ovarian tumor growth, coincident with decreased Ki-67, HMGCR, phosphorylated-Akt and phosphorylated-p42/44 protein expression. Our findings demonstrate that simvastatin may have therapeutic benefit for ovarian cancer treatment and be worthy of further exploration in clinical trials.
Keywords: HMGCR; apoptosis; invasion; ovarian cancer; simvastatin.
Conflict of interest statement
The authors have no potential conflicts of interest to report.
Figures




Similar articles
-
Simvastatin, an HMG-CoA reductase inhibitor, exhibits anti-metastatic and anti-tumorigenic effects in endometrial cancer.Gynecol Oncol. 2014 Aug;134(2):346-55. doi: 10.1016/j.ygyno.2014.05.015. Epub 2014 May 28. Gynecol Oncol. 2014. PMID: 24880141 Free PMC article.
-
Ovarian tumour growth is characterized by mevalonate pathway gene signature in an orthotopic, syngeneic model of epithelial ovarian cancer.Oncotarget. 2016 Jul 26;7(30):47343-47365. doi: 10.18632/oncotarget.10121. Oncotarget. 2016. PMID: 27329838 Free PMC article.
-
Simvastatin-induced breast cancer cell death and deactivation of PI3K/Akt and MAPK/ERK signalling are reversed by metabolic products of the mevalonate pathway.Oncotarget. 2016 Jan 19;7(3):2532-44. doi: 10.18632/oncotarget.6304. Oncotarget. 2016. PMID: 26565813 Free PMC article.
-
The effect of statins on cancer cells--review.Tumour Biol. 2015 Jul;36(7):4889-904. doi: 10.1007/s13277-015-3551-7. Epub 2015 May 23. Tumour Biol. 2015. PMID: 26002574 Review.
-
Role of DNA damage in atherosclerosis--bystander or participant?Biochem Pharmacol. 2011 Oct 1;82(7):693-700. doi: 10.1016/j.bcp.2011.06.025. Epub 2011 Jun 24. Biochem Pharmacol. 2011. PMID: 21726542 Review.
Cited by
-
Simvastatin Overcomes Resistance to Tyrosine Kinase Inhibitors in Patient-derived, Oncogene-driven Lung Adenocarcinoma Models.Mol Cancer Ther. 2024 May 2;23(5):700-710. doi: 10.1158/1535-7163.MCT-23-0458. Mol Cancer Ther. 2024. PMID: 38237027 Free PMC article.
-
Statin use and survival outcomes in endocrine-related gynecologic cancers: A systematic review and meta-analysis.Oncotarget. 2017 Jun 20;8(25):41508-41517. doi: 10.18632/oncotarget.17242. Oncotarget. 2017. PMID: 28489569 Free PMC article. Review.
-
Lovastatin induced Kruppel like factor 2 (KLF2), Kruppel like factor 6 (KLF6) and Ras homolog family member B (RHOB) genes and preferentially led to viability reduction of Cisplatin-resistant cells.Oncotarget. 2017 Nov 16;8(63):106429-106442. doi: 10.18632/oncotarget.22472. eCollection 2017 Dec 5. Oncotarget. 2017. PMID: 29290960 Free PMC article.
-
Antiangiogenic and Proapoptotic Activities of Atorvastatin and Ganoderma lucidum in Tumor Mouse Model via VEGF and Caspase-3 Pathways.Asian Pac J Cancer Prev. 2021 Apr 1;22(4):1095-1104. doi: 10.31557/APJCP.2021.22.4.1095. Asian Pac J Cancer Prev. 2021. PMID: 33906301 Free PMC article.
-
Targeted Mevalonate Pathway and Autophagy in Antitumor Immunotherapy.Curr Cancer Drug Targets. 2024;24(9):890-909. doi: 10.2174/0115680096273730231206054104. Curr Cancer Drug Targets. 2024. PMID: 38275055 Review.
References
-
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA: a cancer journal for clinicians. 2015;65:5–29. - PubMed
-
- Mirandola L, M JC, Cobos E, Bernardini G, Jenkins MR, Kast WM, Chiriva-Internati M. Cancer testis antigens: novel biomarkers and targetable proteins for ovarian cancer. International reviews of immunology. 2011;30:127–137. - PubMed
-
- Robinson E, Nandi M, Wilkinson LL, Arrowsmith DM, Curtis AD, Richardson A. Preclinical evaluation of statins as a treatment for ovarian cancer. Gynecologic oncology. 2013;129:417–424. - PubMed
-
- Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

