Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1
- PMID: 26502902
- PMCID: PMC4623921
- DOI: 10.1186/s12977-015-0216-y
Latent HIV-1 is activated by exosomes from cells infected with either replication-competent or defective HIV-1
Abstract
Background: Completion of HIV life cycle in CD4(+) T lymphocytes needs cell activation. We recently reported that treatment of resting CD4(+) T lymphocytes with exosomes produced by HIV-1 infected cells induces cell activation and susceptibility to HIV replication. Here, we present data regarding the effects of these exosomes on cells latently infected with HIV-1.
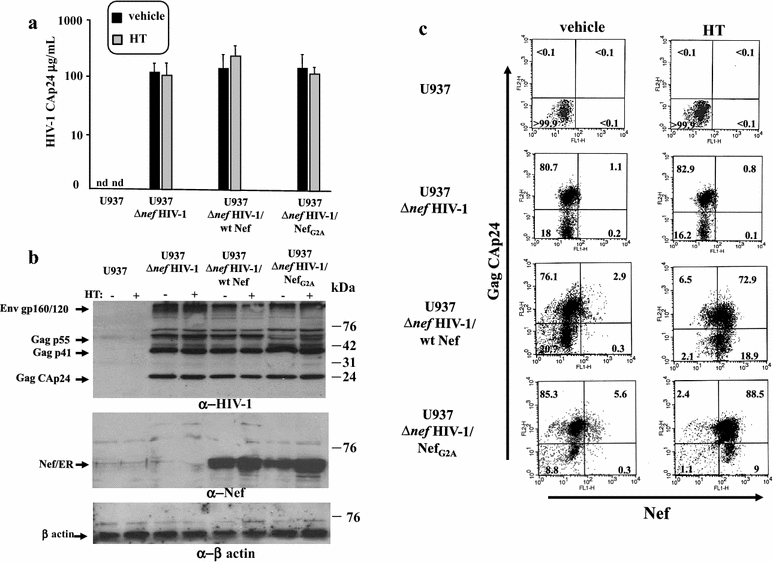
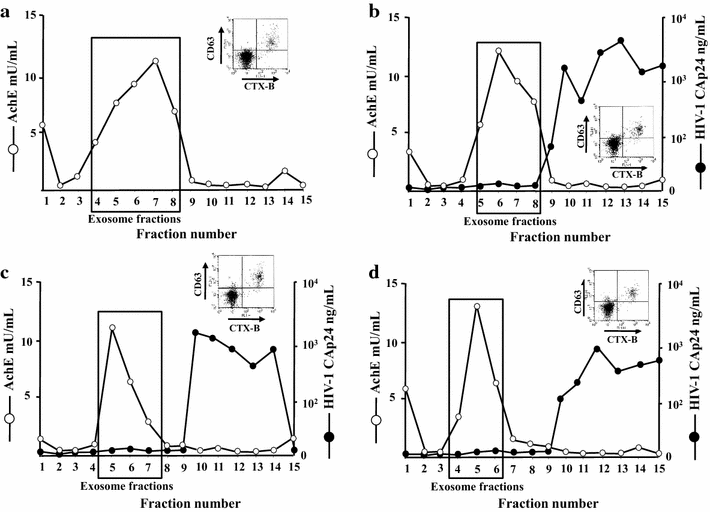
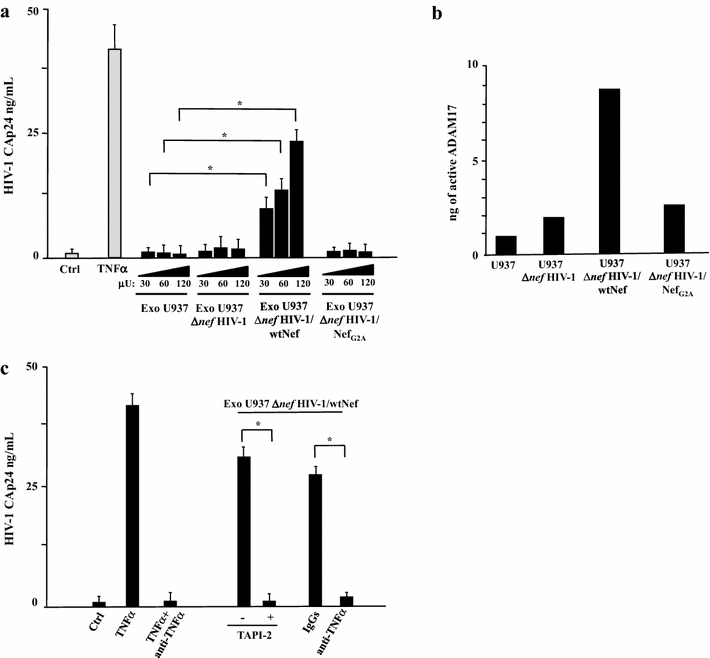
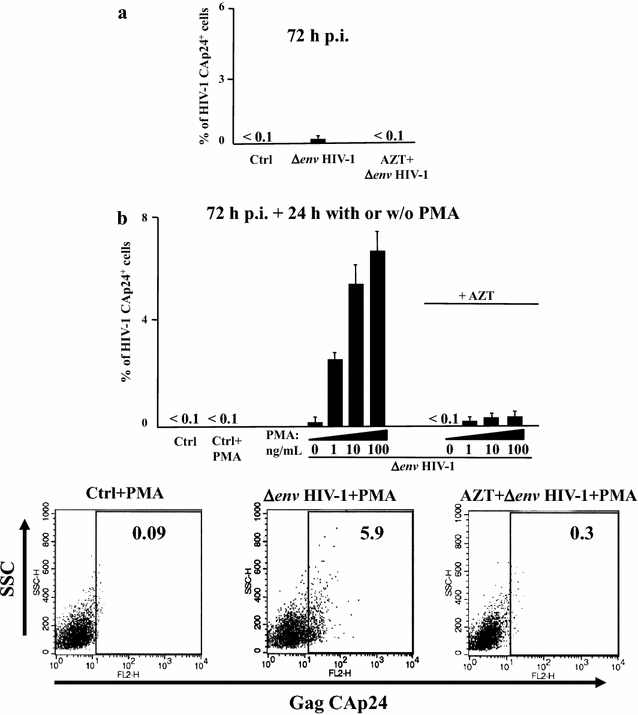
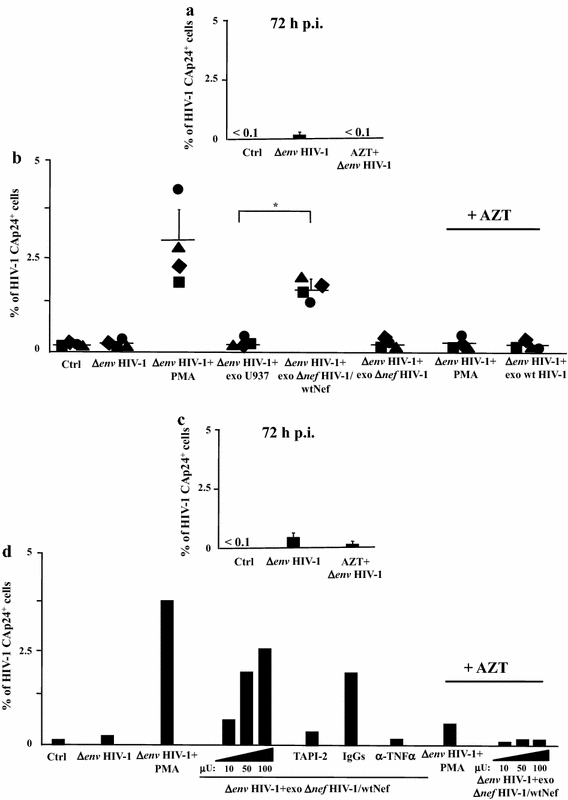
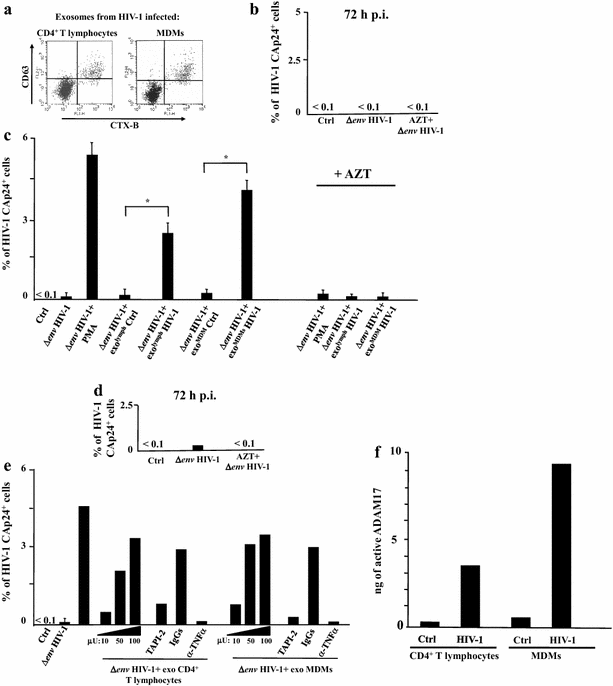
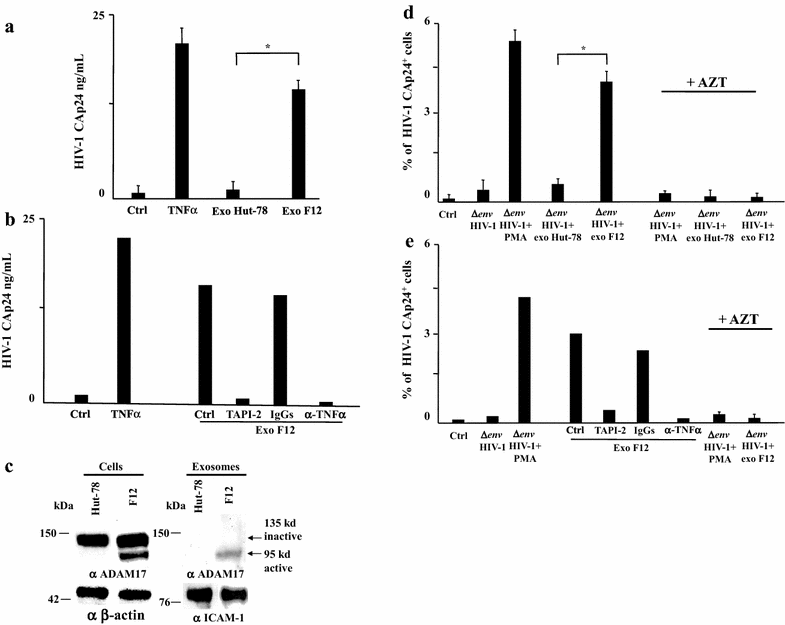
Results: HIV-1 latently infecting U937-derived U1 cells was activated upon challenge with exosomes purified from the supernatant of U937 cells chronically infected with HIV-1. This effect was no more detectable when exosomes from cells infected with HIV-1 strains either nef-deleted or expressing a functionally defective Nef were used, indicating that Nef is the viral determinant of exosome-induced HIV-1 activation. Treatment with either TAPI-2, i.e., a specific inhibitor of the pro-TNFα-processing ADAM17 enzyme, or anti-TNFα Abs abolished HIV-1 activation. Hence, similar to what previously demonstrated for the exosome-mediated activation of uninfected CD4(+) T lymphocytes, the Nef-ADAM17-TNFα axis is part of the mechanism of latent HIV-1 activation. It is noteworthy that these observations have been reproduced using: (1) primary CD4(+) T lymphocytes latently infected with HIV-1; (2) exosomes from both primary CD4(+) T lymphocytes and macrophages acutely infected with HIV-1; (3) co-cultures of HIV-1 acutely infected CD4(+) T lymphocytes and autologous lymphocytes latently infected with HIV-1, and (4) exosomes from cells expressing a defective HIV-1.
Conclusions: Our results strongly suggest that latent HIV-1 can be activated by TNFα released by cells upon ingestion of exosomes released by infected cells, and that this effect depends on the activity of exosome-associated ADAM17. These pieces of evidence shed new light on the mechanism of HIV reactivation in latent reservoirs, and might also be relevant to design new therapeutic interventions focused on HIV eradication.
Figures
Similar articles
-
Exosomes from human immunodeficiency virus type 1 (HIV-1)-infected cells license quiescent CD4+ T lymphocytes to replicate HIV-1 through a Nef- and ADAM17-dependent mechanism.J Virol. 2014 Oct;88(19):11529-39. doi: 10.1128/JVI.01712-14. Epub 2014 Jul 23. J Virol. 2014. PMID: 25056899 Free PMC article.
-
Cell activation and HIV-1 replication in unstimulated CD4+ T lymphocytes ingesting exosomes from cells expressing defective HIV-1.Retrovirology. 2014 Jun 12;11:46. doi: 10.1186/1742-4690-11-46. Retrovirology. 2014. PMID: 24924541 Free PMC article.
-
Trans-dissemination of exosomes from HIV-1-infected cells fosters both HIV-1 trans-infection in resting CD4+ T lymphocytes and reactivation of the HIV-1 reservoir.Arch Virol. 2017 Sep;162(9):2565-2577. doi: 10.1007/s00705-017-3391-4. Epub 2017 May 4. Arch Virol. 2017. PMID: 28474225
-
Reservoirs for HIV-1: mechanisms for viral persistence in the presence of antiviral immune responses and antiretroviral therapy.Annu Rev Immunol. 2000;18:665-708. doi: 10.1146/annurev.immunol.18.1.665. Annu Rev Immunol. 2000. PMID: 10837072 Review.
-
Modulation of apoptosis and viral latency - an axis to be well understood for successful cure of human immunodeficiency virus.J Gen Virol. 2016 Apr;97(4):813-824. doi: 10.1099/jgv.0.000402. Epub 2016 Jan 13. J Gen Virol. 2016. PMID: 26764023 Review.
Cited by
-
Exosomes derived from HIV-1-infected cells promote growth and progression of cancer via HIV TAR RNA.Nat Commun. 2018 Nov 2;9(1):4585. doi: 10.1038/s41467-018-07006-2. Nat Commun. 2018. PMID: 30389917 Free PMC article.
-
The CD8⁺ T Cell-Mediated Immunity Induced by HPV-E6 Uploaded in Engineered Exosomes Is Improved by ISCOMATRIXTM Adjuvant.Vaccines (Basel). 2016 Nov 9;4(4):42. doi: 10.3390/vaccines4040042. Vaccines (Basel). 2016. PMID: 27834857 Free PMC article.
-
Exosomes, microvesicles, and other extracellular vesicles-a Keystone Symposia report.Ann N Y Acad Sci. 2023 May;1523(1):24-37. doi: 10.1111/nyas.14974. Epub 2023 Mar 18. Ann N Y Acad Sci. 2023. PMID: 36961472 Free PMC article.
-
Implications of HIV-1 Nef for "Shock and Kill" Strategies to Eliminate Latent Viral Reservoirs.Viruses. 2018 Nov 30;10(12):677. doi: 10.3390/v10120677. Viruses. 2018. PMID: 30513570 Free PMC article. Review.
-
Detection of HIV-1 and Human Proteins in Urinary Extracellular Vesicles from HIV+ Patients.Adv Virol. 2018 Mar 12;2018:7863412. doi: 10.1155/2018/7863412. eCollection 2018. Adv Virol. 2018. PMID: 29721020 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

