Biomechanical Comparison of Glutaraldehyde-Crosslinked Gelatin Fibrinogen Electrospun Scaffolds to Porcine Coronary Arteries
- PMID: 26501189
- PMCID: PMC4844094
- DOI: 10.1115/1.4031847
Biomechanical Comparison of Glutaraldehyde-Crosslinked Gelatin Fibrinogen Electrospun Scaffolds to Porcine Coronary Arteries
Abstract
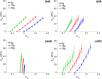
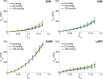
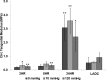
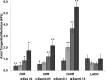
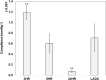
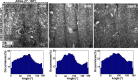
Cardiovascular disease (CVD) is the leading cause of death for Americans. As coronary artery bypass graft surgery (CABG) remains a mainstay of therapy for CVD and native vein grafts are limited by issues of supply and lifespan, an effective readily available tissue-engineered vascular graft (TEVG) for use in CABG would provide drastic improvements in patient care. Biomechanical mismatch between vascular grafts and native vasculature has been shown to be the major cause of graft failure, and therefore, there is need for compliance-matched biocompatible TEVGs for clinical implantation. The current study investigates the biaxial mechanical characterization of acellular electrospun glutaraldehyde (GLUT) vapor-crosslinked gelatin/fibrinogen cylindrical constructs, using a custom-made microbiaxial optomechanical device (MOD). Constructs crosslinked for 2, 8, and 24 hrs are compared to mechanically characterized porcine left anterior descending coronary (LADC) artery. The mechanical response data were used for constitutive modeling using a modified Fung strain energy equation. The results showed that constructs crosslinked for 2 and 8 hrs exhibited circumferential and axial tangential moduli (ATM) similar to that of the LADC. Furthermore, the 8-hrs experimental group was the only one to compliance-match the LADC, with compliance values of 0.0006±0.00018 mm Hg-1 and 0.00071±0.00027 mm Hg-1, respectively. The results of this study show the feasibility of meeting mechanical specifications expected of native arteries through manipulating GLUT vapor crosslinking time. The comprehensive mechanical characterization of cylindrical biopolymer constructs in this study is an important first step to successfully develop a biopolymer compliance-matched TEVG.
Figures





Similar articles
-
Computationally Optimizing the Compliance of a Biopolymer Based Tissue Engineered Vascular Graft.J Biomech Eng. 2016 Jan;138(1):0145051-5. doi: 10.1115/1.4032060. J Biomech Eng. 2016. PMID: 26593773 Free PMC article.
-
Fabrication and characterisation of biomimetic, electrospun gelatin fibre scaffolds for tunica media-equivalent, tissue engineered vascular grafts.Mater Sci Eng C Mater Biol Appl. 2016 Apr 1;61:473-83. doi: 10.1016/j.msec.2015.12.081. Epub 2015 Dec 30. Mater Sci Eng C Mater Biol Appl. 2016. PMID: 26838874
-
A mechanical argument for the differential performance of coronary artery grafts.J Mech Behav Biomed Mater. 2016 Feb;54:93-105. doi: 10.1016/j.jmbbm.2015.09.017. Epub 2015 Sep 21. J Mech Behav Biomed Mater. 2016. PMID: 26437296 Free PMC article.
-
Chemical crosslinking of biopolymeric scaffolds: Current knowledge and future directions of crosslinked engineered bone scaffolds.Int J Biol Macromol. 2018 Feb;107(Pt A):678-688. doi: 10.1016/j.ijbiomac.2017.08.184. Epub 2017 Sep 14. Int J Biol Macromol. 2018. PMID: 28919526 Review.
-
Biomechanical property and modelling of venous wall.Prog Biophys Mol Biol. 2018 Mar;133:56-75. doi: 10.1016/j.pbiomolbio.2017.11.004. Epub 2017 Nov 21. Prog Biophys Mol Biol. 2018. PMID: 29162507 Review.
Cited by
-
Modulating smooth muscle cell response by the release of TGFβ2 from tubular scaffolds for vascular tissue engineering.J Control Release. 2019 Apr 10;299:44-52. doi: 10.1016/j.jconrel.2019.02.024. Epub 2019 Feb 20. J Control Release. 2019. PMID: 30797003 Free PMC article.
-
Stabilisation of Collagen Sponges by Glutaraldehyde Vapour Crosslinking.Int J Biomater. 2017;2017:8947823. doi: 10.1155/2017/8947823. Epub 2017 May 9. Int J Biomater. 2017. PMID: 28572823 Free PMC article.
-
Surface Modification of Electrospun Scaffolds for Endothelialization of Tissue-Engineered Vascular Grafts Using Human Cord Blood-Derived Endothelial Cells.J Clin Med. 2019 Feb 4;8(2):185. doi: 10.3390/jcm8020185. J Clin Med. 2019. PMID: 30720769 Free PMC article.
-
Corrugated nanofiber tissue-engineered vascular graft to prevent kinking for arteriovenous shunts in an ovine model.JVS Vasc Sci. 2020 Apr 11;1:100-108. doi: 10.1016/j.jvssci.2020.03.003. eCollection 2020. JVS Vasc Sci. 2020. PMID: 34617042 Free PMC article.
-
Synthesis and In Vitro Testing of YVO4:Eu3+@silica-NH-GDA-IgG Bio-Nano Complexes for Labelling MCF-7 Breast Cancer Cells.Molecules. 2022 Dec 29;28(1):280. doi: 10.3390/molecules28010280. Molecules. 2022. PMID: 36615474 Free PMC article.
References
-
- Go, A. S. , Mozaffarian, D. , Roger, V. L. , Benjamin, E. J. , Berry, J. D. , Blaha, M. J. , Dai, S. , Ford, E. S. , Fox, C. S. , Franco, S. , Fullerton, H. J. , Gillespie, C. , Hailpern, S. M. , Heit, J. A. , Howard, V. J. , Huffman, M. D. , Judd, S. E. , Kissela, B. M. , Kittner, S. J. , Lackland, D. T. , Lichtman, J. H. , Lisabeth, L. D. , Mackey, R. H. , Magid, D. J. , Marcus, G. M. , Marelli, A. , Matchar, D. B. , McGuire, D. K. , Mohler, E. R. , 3rd, Moy, C. S. , Mussolino, M. E. , Neumar, R. W. , Nichol, G. , Pandey, D. K. , Paynter, N. P. , Reeves, M. J. , Sorlie, P. D. , Stein, J. , Towfighi, A. , Turan, T. N. , Virani, S. S. , Wong, N. D. , Woo, D. , and Turner, M. B. , 2014, “ Heart Disease and Stroke Statistics—2014 Update: A Report From the American Heart Association,” Circulation, 129(3), pp. e28–e292.10.1161/01.cir.0000441139.02102.80 - DOI - PMC - PubMed
-
- He, J. , Qin, T. , Liu, Y. , Li, X. , Li, D. , and Jin, Z. , 2014, “ Electrospinning of Nanofibrous Scaffolds With Continuous Structure and Material Gradients,” Mater. Lett., 137, pp. 393–397.10.1016/j.matlet.2014.09.045 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

