TRIM21: a cytosolic Fc receptor with broad antibody isotype specificity
- PMID: 26497531
- PMCID: PMC4670481
- DOI: 10.1111/imr.12363
TRIM21: a cytosolic Fc receptor with broad antibody isotype specificity
Abstract
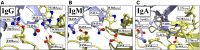
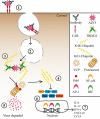
Antibodies are key molecules in the fight against infections. Although previously thought to mediate protection solely in the extracellular environment, recent research has revealed that antibody-mediated protection extends to the cytosolic compartment of cells. This postentry viral defense mechanism requires binding of the antibody to a cytosolic Fc receptor named tripartite motif containing 21 (TRIM21). In contrast to other Fc receptors, TRIM21 shows remarkably broad isotype specificity as it does not only bind IgG but also IgM and IgA. When viral pathogens coated with these antibody isotypes enter the cytosol, TRIM21 is rapidly recruited and efficient neutralization occurs before the virus has had the time to replicate. In addition, inflammatory signaling is induced. As such, TRIM21 acts as a cytosolic sensor that engages antibodies that have failed to protect against infection in the extracellular environment. Here, we summarize our current understanding of how TRIM21 orchestrates humoral immunity in the cytosolic environment.
Keywords: Fc-receptor; TRIM21; antibodies; intracellular immunity; isotype; virus.
© 2015 The Authors. Immunological Reviews Published by John Wiley & Sons Ltd.
Figures

Similar articles
-
Translocalized IgA mediates neutralization and stimulates innate immunity inside infected cells.Proc Natl Acad Sci U S A. 2014 Sep 16;111(37):13463-8. doi: 10.1073/pnas.1410980111. Epub 2014 Aug 28. Proc Natl Acad Sci U S A. 2014. PMID: 25169018 Free PMC article.
-
Swine TRIM21 restricts FMDV infection via an intracellular neutralization mechanism.Antiviral Res. 2016 Mar;127:32-40. doi: 10.1016/j.antiviral.2016.01.004. Epub 2016 Jan 14. Antiviral Res. 2016. PMID: 26777733
-
Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21).Proc Natl Acad Sci U S A. 2010 Nov 16;107(46):19985-90. doi: 10.1073/pnas.1014074107. Epub 2010 Nov 2. Proc Natl Acad Sci U S A. 2010. PMID: 21045130 Free PMC article.
-
TRIM21-From Intracellular Immunity to Therapy.Front Immunol. 2019 Aug 28;10:2049. doi: 10.3389/fimmu.2019.02049. eCollection 2019. Front Immunol. 2019. PMID: 31555278 Free PMC article. Review.
-
Coordinated Neutralization and Immune Activation by the Cytosolic Antibody Receptor TRIM21.J Virol. 2016 Apr 29;90(10):4856-4859. doi: 10.1128/JVI.00050-16. Print 2016 May 15. J Virol. 2016. PMID: 26937031 Free PMC article. Review.
Cited by
-
Immune recruitment or suppression by glycan engineering of endogenous and therapeutic antibodies.Biochim Biophys Acta. 2016 Aug;1860(8):1655-68. doi: 10.1016/j.bbagen.2016.04.016. Epub 2016 Apr 20. Biochim Biophys Acta. 2016. PMID: 27105835 Free PMC article. Review.
-
Clinical Significance of Different Profiles of anti-Ro Antibodies in Connective Tissue Diseases.J Immunol Res. 2023 Jan 25;2023:9195157. doi: 10.1155/2023/9195157. eCollection 2023. J Immunol Res. 2023. PMID: 36741231 Free PMC article.
-
Functional diversification of innate and inflammatory immune responses mediated by antibody fragment crystallizable activities against SARS-CoV-2.iScience. 2024 Apr 11;27(5):109703. doi: 10.1016/j.isci.2024.109703. eCollection 2024 May 17. iScience. 2024. PMID: 38706870 Free PMC article.
-
Fcγ Receptor Function and the Design of Vaccination Strategies.Immunity. 2017 Aug 15;47(2):224-233. doi: 10.1016/j.immuni.2017.07.009. Immunity. 2017. PMID: 28813656 Free PMC article. Review.
-
TRIM21 and Fc-engineered antibodies: decoding its complex antibody binding mode with implications for viral neutralization.Front Immunol. 2024 Jun 12;15:1401471. doi: 10.3389/fimmu.2024.1401471. eCollection 2024. Front Immunol. 2024. PMID: 38938560 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

