CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression
- PMID: 26493679
- PMCID: PMC5341230
- DOI: 10.1111/bph.13372
CREB, AP-1, ternary complex factors and MAP kinases connect transient receptor potential melastatin-3 (TRPM3) channel stimulation with increased c-Fos expression
Abstract
Background and purpose: The rise in intracellular Ca(2+) stimulates the expression of the transcription factor c-Fos. Depending on the mode of entry of Ca(2+) into the cytosol, distinct signal transducers and transcription factors are required. Here, we have analysed the signalling pathway connecting a Ca(2+) influx via activation of transient receptor potential melastatin-3 (TRPM3) channels with enhanced c-Fos expression.
Experimental approach: Transcription of c-Fos promoter/reporter genes that were integrated into the chromatin via lentiviral gene transfer was analysed in HEK293 cells overexpressing TRPM3. The transcriptional activation potential of c-Fos was measured using a GAL4-c-Fos fusion protein.
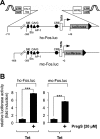
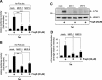
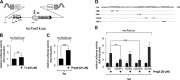
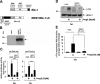
Key results: The signalling pathway connecting TRPM3 stimulation with enhanced c-Fos expression requires the activation of MAP kinases. On the transcriptional level, three Ca(2+) -responsive elements, the cAMP-response element and the binding sites for the serum response factor (SRF) and AP-1, are essential for the TRPM3-mediated stimulation of the c-Fos promoter. Ternary complex factors are additionally involved in connecting TRPM3 stimulation with the up-regulation of c-Fos expression. Stimulation of TRPM3 channels also increases the transcriptional activation potential of c-Fos.
Conclusions and implications: Signalling molecules involved in connecting TRPM3 with the c-Fos gene are MAP kinases and the transcription factors CREB, SRF, AP-1 and ternary complex factors. As c-Fos constitutes, together with other basic region leucine zipper transcription factors, the AP-1 transcription factor complex, the results of this study explain TRPM3-induced activation of AP-1 and connects TRPM3 with the biological functions regulated by AP-1.
© 2015 The British Pharmacological Society.
Figures




Similar articles
-
Transient receptor potential melastatin-3 (TRPM3)-induced activation of AP-1 requires Ca2+ ions and the transcription factors c-Jun, ATF2, and ternary complex factor.Mol Pharmacol. 2015 Apr;87(4):617-28. doi: 10.1124/mol.114.095695. Epub 2015 Jan 9. Mol Pharmacol. 2015. PMID: 25576487
-
Stimulation of transient receptor potential M3 (TRPM3) channels increases interleukin-8 gene promoter activity involving AP-1 and extracellular signal-regulated protein kinase.Cytokine. 2018 Mar;103:133-141. doi: 10.1016/j.cyto.2017.09.020. Epub 2017 Oct 2. Cytokine. 2018. PMID: 28982580
-
Extracellular Signal-Regulated Protein Kinase, c-Jun N-Terminal Protein Kinase, and Calcineurin Regulate Transient Receptor Potential M3 (TRPM3) Induced Activation of AP-1.J Cell Biochem. 2017 Aug;118(8):2409-2419. doi: 10.1002/jcb.25904. Epub 2017 Apr 25. J Cell Biochem. 2017. PMID: 28112420
-
Transient receptor potential TRPM3 channels: Pharmacology, signaling, and biological functions.Pharmacol Res. 2017 Oct;124:92-99. doi: 10.1016/j.phrs.2017.07.014. Epub 2017 Jul 16. Pharmacol Res. 2017. PMID: 28720517 Review.
-
Signal transduction via TRPM3 channels in pancreatic β-cells.J Mol Endocrinol. 2013 Apr 23;50(3):R75-83. doi: 10.1530/JME-12-0237. Print 2013 Jun. J Mol Endocrinol. 2013. PMID: 23511953 Review.
Cited by
-
TRPM3_miR-204: a complex locus for eye development and disease.Hum Genomics. 2020 Feb 18;14(1):7. doi: 10.1186/s40246-020-00258-4. Hum Genomics. 2020. PMID: 32070426 Free PMC article. Review.
-
Impaired calcium mobilization in natural killer cells from chronic fatigue syndrome/myalgic encephalomyelitis patients is associated with transient receptor potential melastatin 3 ion channels.Clin Exp Immunol. 2017 Feb;187(2):284-293. doi: 10.1111/cei.12882. Epub 2016 Nov 23. Clin Exp Immunol. 2017. PMID: 27727448 Free PMC article.
-
Ca2+ Microdomains, Calcineurin and the Regulation of Gene Transcription.Cells. 2021 Apr 12;10(4):875. doi: 10.3390/cells10040875. Cells. 2021. PMID: 33921430 Free PMC article. Review.
-
The Impact of Steroid Activation of TRPM3 on Spontaneous Activity in the Developing Retina.eNeuro. 2020 Apr 22;7(2):ENEURO.0175-19.2020. doi: 10.1523/ENEURO.0175-19.2020. Print 2020 Mar/Apr. eNeuro. 2020. PMID: 32238415 Free PMC article.
-
circPRRC2A promotes angiogenesis and metastasis through epithelial-mesenchymal transition and upregulates TRPM3 in renal cell carcinoma.Theranostics. 2020 Mar 15;10(10):4395-4409. doi: 10.7150/thno.43239. eCollection 2020. Theranostics. 2020. PMID: 32292503 Free PMC article.
References
-
- Cahill MA, Janknecht R, Nordheim A (1995). Signal uptake by the c‐fos serum response element In: Baeuerle PA. Inducible Gene Expression, Vol. 2 Birkhäuser: Boston/Basel/Berlin, pp. 39–72.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

