Oligodendrocyte Development and Plasticity
- PMID: 26492571
- PMCID: PMC4743079
- DOI: 10.1101/cshperspect.a020453
Oligodendrocyte Development and Plasticity
Abstract
Oligodendrocyte precursor cells (OPCs) originate in the ventricular zones (VZs) of the brain and spinal cord and migrate throughout the developing central nervous system (CNS) before differentiating into myelinating oligodendrocytes (OLs). It is not known whether OPCs or OLs from different parts of the VZ are functionally distinct. OPCs persist in the postnatal CNS, where they continue to divide and generate myelinating OLs at a decreasing rate throughout adult life in rodents. Adult OPCs respond to injury or disease by accelerating their cell cycle and increasing production of OLs to replace lost myelin. They also form synapses with unmyelinated axons and respond to electrical activity in those axons by generating more OLs and myelin locally. This experience-dependent "adaptive" myelination is important in some forms of plasticity and learning, for example, motor learning. We review the control of OL lineage development, including OL population dynamics and adaptive myelination in the adult CNS.
Copyright © 2016 Cold Spring Harbor Laboratory Press; all rights reserved.
Figures
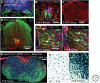
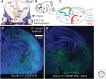
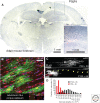
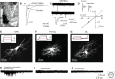
Similar articles
-
Cleavage of VAMP2/3 Affects Oligodendrocyte Lineage Development in the Developing Mouse Spinal Cord.J Neurosci. 2023 Sep 27;43(39):6592-6608. doi: 10.1523/JNEUROSCI.2206-21.2023. Epub 2023 Aug 24. J Neurosci. 2023. PMID: 37620160 Free PMC article.
-
Glutamate versus GABA in neuron-oligodendroglia communication.Glia. 2019 Nov;67(11):2092-2106. doi: 10.1002/glia.23618. Epub 2019 Apr 7. Glia. 2019. PMID: 30957306 Review.
-
Life-long oligodendrocyte development and plasticity.Semin Cell Dev Biol. 2021 Aug;116:25-37. doi: 10.1016/j.semcdb.2021.02.004. Epub 2021 Mar 16. Semin Cell Dev Biol. 2021. PMID: 33741250 Free PMC article.
-
From precursors to myelinating oligodendrocytes: contribution of intrinsic and extrinsic factors to white matter plasticity in the adult brain.Neuroscience. 2014 Jun 6;269:343-66. doi: 10.1016/j.neuroscience.2014.03.063. Epub 2014 Apr 8. Neuroscience. 2014. PMID: 24721734 Review.
-
Oligodendrocyte dynamics in the healthy adult CNS: evidence for myelin remodeling.Neuron. 2013 Mar 6;77(5):873-85. doi: 10.1016/j.neuron.2013.01.006. Neuron. 2013. PMID: 23473318 Free PMC article.
Cited by
-
Palmitoylethanolamide and White Matter Lesions: Evidence for Therapeutic Implications.Biomolecules. 2022 Aug 27;12(9):1191. doi: 10.3390/biom12091191. Biomolecules. 2022. PMID: 36139030 Free PMC article. Review.
-
Ontogeny of adult neural stem cells in the mammalian brain.Curr Top Dev Biol. 2021;142:67-98. doi: 10.1016/bs.ctdb.2020.11.002. Epub 2020 Dec 17. Curr Top Dev Biol. 2021. PMID: 33706926 Free PMC article. Review.
-
The emerging role of galectins in (re)myelination and its potential for developing new approaches to treat multiple sclerosis.Cell Mol Life Sci. 2020 Apr;77(7):1289-1317. doi: 10.1007/s00018-019-03327-7. Epub 2019 Oct 18. Cell Mol Life Sci. 2020. PMID: 31628495 Free PMC article. Review.
-
Oligodendrocytes in intracerebral hemorrhage.CNS Neurosci Ther. 2019 Oct;25(10):1075-1084. doi: 10.1111/cns.13193. Epub 2019 Aug 14. CNS Neurosci Ther. 2019. PMID: 31410988 Free PMC article. Review.
-
Motor Exit Point (MEP) Glia: Novel Myelinating Glia That Bridge CNS and PNS Myelin.Front Cell Neurosci. 2018 Oct 2;12:333. doi: 10.3389/fncel.2018.00333. eCollection 2018. Front Cell Neurosci. 2018. PMID: 30356886 Free PMC article. Review.
References
-
- Agius E, Soukkarieh C, Danesin C, Kan P, Takebayashi H, Soula C, Cochard P. 2004. Converse control of oligodendrocyte and astrocyte lineage development by Sonic hedgehog in the chick spinal cord. Dev Biol 270: 308–321. - PubMed
-
- Ahlgren SC, Wallace H, Bishop J, Neophytou C, Raff MC. 1997. Effects of thyroid hormone on embryonic oligodendrocyte precursor cell development in vivo and in vitro. Mol Cell Neurosci 9: 420–432. - PubMed
-
- Anderson ES, Bjartmar C, Westermark G, Hildebrand C. 1999. Molecular heterogeneity of oligodendrocytes in chicken white matter. Glia 27: 15–21. - PubMed
-
- Arnett HA, Fancy SP, Alberta JA, Zhao C, Plant SR, Kaing S, Raine CS, Rowitch DH, Franklin RJ, Stiles CD. 2004. bHLH transcription factor Olig1 is required to repair demyelinated lesions in the CNS. Science 306: 2111–2115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
