64Cu-DOTA as a surrogate positron analog of Gd-DOTA for cardiac fibrosis detection with PET: pharmacokinetic study in a rat model of chronic MI
- PMID: 26488428
- PMCID: PMC4689643
- DOI: 10.1097/MNM.0000000000000417
64Cu-DOTA as a surrogate positron analog of Gd-DOTA for cardiac fibrosis detection with PET: pharmacokinetic study in a rat model of chronic MI
Abstract
Objectives: The aim of this study was to investigate the pharmacokinetics of (64)Cu-DOTA (1,4,7,10-azacyclododecane-N,N',N'',N'''-tetraacetic acid), a positron surrogate analog of the late gadolinium (Gd)-enhancement cardiac magnetic resonance agent, Gd-DOTA, in a rat model of chronic myocardial infarction (MI) and its microdistribution in the cardiac fibrosis by autoradiography.
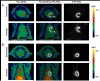
Methods: DOTA was labeled with (64)Cu-acetate. CD rats (n=5) with MI by left anterior descending coronary artery ligation and normal rats (n=6) were injected intravenously with (64)Cu-DOTA (18.5 MBq, 0.02 mmol DOTA/kg). Dynamic PET imaging was performed for 60 min after injection. (18)F-Fluorodeoxyglucose ([(18)F]-FDG) PET imaging was performed to identify the viable myocardium. For the region of interest analysis, the (64)Cu-DOTA PET image was coregistered to the [(18)F]-FDG PET image. To validate the PET images, slices of heart samples from the base to the apex were analyzed using autoradiography and by histological staining with Masson's trichrome.
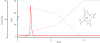
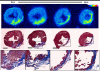
Results: (64)Cu-DOTA was rapidly taken up in the infarct area. The time-activity curves demonstrated that (64)Cu-DOTA concentrations in the blood, fibrotic tissue, and perfusion-rich organs peaked within a minute post injection; thereafter, it was rapidly washed out in parallel with blood clearance and excreted through the renal system. The blood clearance curve was biphasic, with a distribution half-life of less than 3 min and an elimination half-life of ∼21.8 min. The elimination half-life of (64)Cu-DOTA from the focal fibrotic tissue (∼22.4 min) and the remote myocardium (∼20.1 min) was similar to the blood elimination half-life. Consequently, the uptake ratios of focal fibrosis-to-blood and remote myocardium-to-blood remained stable for the time period between 10 and 60 min. The corresponding ratios obtained from images acquired from 30 to 60 min were 1.09 and 0.59, respectively, indicating that the concentration of (64)Cu-DOTA in the focal fibrosis was 1.85 (1.09/0.59) times greater than that in the remote myocardium. Thus, this finding indicates that the extracellular volume fraction was 1.85 times greater in the focal fibrosis than in the remote myocardium. The accumulation of (64)Cu-DOTA in fibrotic tissue was further supported by autoradiography and histology images. The autoradiography images of (64)Cu-DOTA in the fibrotic tissues were qualitatively superimposed over the histology images of the fibrotic tissues. The histology images of the infarct areas were characterized by a heterogeneous distribution of thin bands of fibrotic collagen, myocytes, and expanded extracellular space.
Conclusion: (64)Cu-DOTA is a useful surrogate positron analog of Gd-DOTA, enabling quantitative measurement of the uptake values in fibrotic tissues by dynamic PET imaging and calculation of the extracellular volume fractions of the fibrotic tissues. At a microscopic level, the distribution of (64)Cu-DOTA is nonuniform, corresponding to the heterogeneous distribution of expanded extracellular space in the setting of MI.
Figures

Similar articles
-
Pharmacokinetics and microbiodistribution of 64Cu-labeled collagen-binding peptides in chronic myocardial infarction.Nucl Med Commun. 2016 Dec;37(12):1306-1317. doi: 10.1097/MNM.0000000000000590. Nucl Med Commun. 2016. PMID: 27623511 Free PMC article.
-
68Ga-DOTA chelate, a novel imaging agent for assessment of myocardial perfusion and infarction detection in a rodent model.J Nucl Cardiol. 2020 Jun;27(3):891-898. doi: 10.1007/s12350-019-01752-6. Epub 2019 May 29. J Nucl Cardiol. 2020. PMID: 31144229 Free PMC article.
-
Imaging of VEGF receptor in a rat myocardial infarction model using PET.J Nucl Med. 2008 Apr;49(4):667-73. doi: 10.2967/jnumed.107.040576. J Nucl Med. 2008. PMID: 18375924 Free PMC article.
-
Molecular Probes for Imaging Fibrosis and Fibrogenesis.Chemistry. 2019 Jan 24;25(5):1128-1141. doi: 10.1002/chem.201801578. Epub 2018 Nov 21. Chemistry. 2019. PMID: 30014529 Free PMC article. Review.
-
Radiotracers for Imaging of Fibrosis: Advances during the Last Two Decades and Future Directions.Pharmaceuticals (Basel). 2023 Nov 1;16(11):1540. doi: 10.3390/ph16111540. Pharmaceuticals (Basel). 2023. PMID: 38004406 Free PMC article. Review.
Cited by
-
Pharmacokinetics and microbiodistribution of 64Cu-labeled collagen-binding peptides in chronic myocardial infarction.Nucl Med Commun. 2016 Dec;37(12):1306-1317. doi: 10.1097/MNM.0000000000000590. Nucl Med Commun. 2016. PMID: 27623511 Free PMC article.
-
Noninvasive assessment of renal fibrosis by magnetic resonance imaging and ultrasound techniques.Transl Res. 2019 Jul;209:105-120. doi: 10.1016/j.trsl.2019.02.009. Epub 2019 Apr 22. Transl Res. 2019. PMID: 31082371 Free PMC article. Review.
-
Explicit measurement of multi-tracer arterial input function for PET imaging using blood sampling spectroscopy.EJNMMI Phys. 2020 Feb 6;7(1):7. doi: 10.1186/s40658-020-0277-4. EJNMMI Phys. 2020. PMID: 32030519 Free PMC article.
-
Radionuclide Cisternography with [64Cu]Cu-DOTA.Pharmaceuticals (Basel). 2023 Sep 7;16(9):1269. doi: 10.3390/ph16091269. Pharmaceuticals (Basel). 2023. PMID: 37765077 Free PMC article.
References
-
- Coelho-Filho OR, Rickers C, Kwong RY, Jerosch-Herold M. MR myocardial perfusion imaging. Radiology. 2013;266:701–715. - PubMed
-
- Herborn CU, Watkins DM, Baumann S, Robert P, Corot C, Runge VM. Contrast-enhanced magnetic resonance angiography: P792 blood pool agent versus Gd-DOTA in rabbits at 3.0 T versus 1.5 T. Invest Radiol. 2007;42:622–628. - PubMed
-
- Ovize M, Revel D, de Lorgeril M, Pichard JB, Dandis G, Delaye J, et al. Quantitation of reperfused myocardial infarction by Gd-DOTA-enhanced magnetic resonance imaging. An experimental study. Invest Radiol. 1991;26:1065–1070. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

