Bridging the Gap Between Validation and Implementation of Non-Animal Veterinary Vaccine Potency Testing Methods
- PMID: 26486625
- PMCID: PMC4513470
- DOI: 10.3390/ani1040414
Bridging the Gap Between Validation and Implementation of Non-Animal Veterinary Vaccine Potency Testing Methods
Abstract
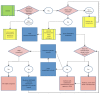
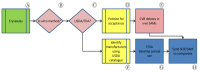
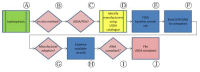
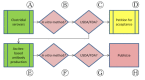
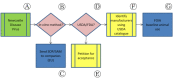
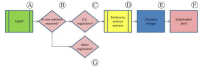
In recent years, technologically advanced high-throughput techniques have been developed that replace, reduce or refine animal use in vaccine quality control tests. Following validation, these tests are slowly being accepted for use by international regulatory authorities. Because regulatory acceptance itself has not guaranteed that approved humane methods are adopted by manufacturers, various organizations have sought to foster the preferential use of validated non-animal methods by interfacing with industry and regulatory authorities. After noticing this gap between regulation and uptake by industry, we began developing a paradigm that seeks to narrow the gap and quicken implementation of new replacement, refinement or reduction guidance. A systematic analysis of our experience in promoting the transparent implementation of validated non-animal vaccine potency assays has led to the refinement of our paradigmatic process, presented here, by which interested parties can assess the local regulatory acceptance of methods that reduce animal use and integrate them into quality control testing protocols, or ensure the elimination of peripheral barriers to their use, particularly for potency and other tests carried out on production batches.
Keywords: implementation; laboratory animals; non-animal; potency; regulatory testing; safety; vaccine.
Figures
Similar articles
-
Three Rs potential in the development and quality control of immunobiologicals.ALTEX. 2001;18 Suppl 1:13-47. ALTEX. 2001. PMID: 11854853
-
Potency testing of veterinary vaccines: the way from in vivo to in vitro.Biologicals. 2012 Jan;40(1):100-6. doi: 10.1016/j.biologicals.2011.10.004. Epub 2011 Nov 9. Biologicals. 2012. PMID: 22075457
-
Recent progress and future directions for reduction, refinement, and replacement of animal use in veterinary vaccine potency and safety testing: a report from the 2010 NICEATM-ICCVAM International Vaccine Workshop.Dev Biol (Basel). 2012;134:9-21. Dev Biol (Basel). 2012. PMID: 22888590
-
Regulatory Acceptance of Alternative Methods in the Development and Approval of Pharmaceuticals.Adv Exp Med Biol. 2016;856:33-64. doi: 10.1007/978-3-319-33826-2_3. Adv Exp Med Biol. 2016. PMID: 27671719 Review.
-
Implementation of the 3Rs (refinement, reduction, and replacement): validation and regulatory acceptance considerations for alternative toxicological test methods.ILAR J. 2002;43 Suppl:S85-94. doi: 10.1093/ilar.43.suppl_1.s85. ILAR J. 2002. PMID: 12388858 Review.
References
-
- Halder M. Three Rs potential in the development and quality control of immunobiologicals. ALTEX. 2001;18:13–47. - PubMed
-
- Stern A.M., Markel H. The history of vaccines and immunization: Familiar patterns, new challenges. Health Aff. (Millwood) 2005;24:611–621. - PubMed
-
- D'Argenio D.A., Wilson C.B. A decade of vaccines: Integrating immunology and vaccinology for rational vaccine design. Immunity. 2010;33:437–440. - PubMed
-
- Lombard M., Pastoret P.P., Moulin A.M. A brief history of vaccines and vaccination. Rev. Sci. Tech. 2007;26:29–48. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

