Novel angiogenin mutants with increased cytotoxicity enhance the depletion of pro-inflammatory macrophages and leukemia cells ex vivo
- PMID: 26472728
- PMCID: PMC11028715
- DOI: 10.1007/s00262-015-1763-8
Novel angiogenin mutants with increased cytotoxicity enhance the depletion of pro-inflammatory macrophages and leukemia cells ex vivo
Abstract
Immunotoxins are fusion proteins that combine a targeting component such as an antibody fragment or ligand with a cytotoxic effector component that induces apoptosis in specific cell populations displaying the corresponding antigen or receptor. Human cytolytic fusion proteins (hCFPs) are less immunogenic than conventional immunotoxins because they contain human pro-apoptotic enzymes as effectors. However, one drawback of hCFPs is that target cells can protect themselves by expressing endogenous inhibitor proteins. Inhibitor-resistant enzyme mutants that maintain their cytotoxic activity are therefore promising effector domain candidates. We recently developed potent variants of the human ribonuclease angiogenin (Ang) that were either more active than the wild-type enzyme or less susceptible to inhibition because of their lower affinity for the ribonuclease inhibitor RNH1. However, combining the mutations was unsuccessful because although the enzyme retained its higher activity, its susceptibility to RNH1 reverted to wild-type levels. We therefore used molecular dynamic simulations to determine, at the atomic level, why the affinity for RNH1 reverted, and we developed strategies based on the introduction of further mutations to once again reduce the affinity of Ang for RNH1 while retaining its enhanced activity. We were able to generate a novel Ang variant with remarkable in vitro cytotoxicity against HL-60 cells and pro-inflammatory macrophages. We also demonstrated the pro-apoptotic potential of Ang-based hCFPs on cells freshly isolated from leukemia patients.
Keywords: Angiogenin; Human cytolytic fusion protein; Leukemia; RNH1; Site-directed mutagenesis; Targeted therapy.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures
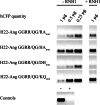
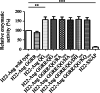

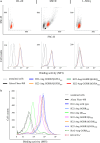
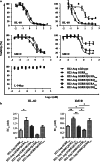
Similar articles
-
Designing the Sniper: Improving Targeted Human Cytolytic Fusion Proteins for Anti-Cancer Therapy via Molecular Simulation.Biomedicines. 2017 Feb 17;5(1):9. doi: 10.3390/biomedicines5010009. Biomedicines. 2017. PMID: 28536352 Free PMC article. Review.
-
Angiogenin mutants as novel effector molecules for the generation of fusion proteins with increased cytotoxic potential.J Immunother. 2015 Apr;38(3):85-95. doi: 10.1097/CJI.0000000000000053. J Immunother. 2015. PMID: 25710248
-
Ribonuclease/angiogenin inhibitor 1 regulates stress-induced subcellular localization of angiogenin to control growth and survival.J Cell Sci. 2013 Sep 15;126(Pt 18):4308-19. doi: 10.1242/jcs.134551. Epub 2013 Jul 10. J Cell Sci. 2013. PMID: 23843625 Free PMC article.
-
Human angiogenin is a potent cytotoxin in the absence of ribonuclease inhibitor.RNA. 2018 Aug;24(8):1018-1027. doi: 10.1261/rna.065516.117. Epub 2018 May 10. RNA. 2018. PMID: 29748193 Free PMC article.
-
Angiogenin (ANG)-Ribonuclease Inhibitor (RNH1) System in Protein Synthesis and Disease.Int J Mol Sci. 2021 Jan 28;22(3):1287. doi: 10.3390/ijms22031287. Int J Mol Sci. 2021. PMID: 33525475 Free PMC article. Review.
Cited by
-
Designing the Sniper: Improving Targeted Human Cytolytic Fusion Proteins for Anti-Cancer Therapy via Molecular Simulation.Biomedicines. 2017 Feb 17;5(1):9. doi: 10.3390/biomedicines5010009. Biomedicines. 2017. PMID: 28536352 Free PMC article. Review.
-
Advances in epidermal growth factor receptor specific immunotherapy: lessons to be learned from armed antibodies.Oncotarget. 2020 Sep 22;11(38):3531-3557. doi: 10.18632/oncotarget.27730. eCollection 2020 Sep 22. Oncotarget. 2020. PMID: 33014289 Free PMC article. Review.
-
CD64-directed microtubule associated protein tau kills leukemic blasts ex vivo.Oncotarget. 2016 Oct 11;7(41):67166-67174. doi: 10.18632/oncotarget.11568. Oncotarget. 2016. PMID: 27564103 Free PMC article.
-
Updates in the Development of ImmunoRNases for the Selective Killing of Tumor Cells.Biomedicines. 2018 Mar 5;6(1):28. doi: 10.3390/biomedicines6010028. Biomedicines. 2018. PMID: 29510557 Free PMC article. Review.
-
Targeted human cytolytic fusion proteins at the cutting edge: harnessing the apoptosis-inducing properties of human enzymes for the selective elimination of tumor cells.Oncotarget. 2019 Jan 25;10(8):897-915. doi: 10.18632/oncotarget.26618. eCollection 2019 Jan 25. Oncotarget. 2019. PMID: 30783518 Free PMC article. Review.
References
-
- Weidle UH, Georges G, Brinkmann U. Fully human targeted cytotoxic fusion proteins: new anticancer agents on the horizon. Cancer Genomics Proteomics. 2012;9:119–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

