The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II
- PMID: 26468525
- PMCID: PMC4702578
- DOI: 10.1128/JVI.02183-15
The Missing Link in Epstein-Barr Virus Immune Evasion: the BDLF3 Gene Induces Ubiquitination and Downregulation of Major Histocompatibility Complex Class I (MHC-I) and MHC-II
Abstract
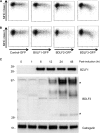
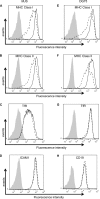
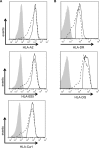
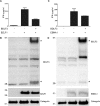
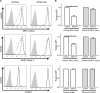
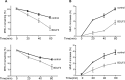
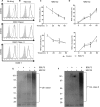
The ability of Epstein-Barr virus (EBV) to spread and persist in human populations relies on a balance between host immune responses and EBV immune evasion. CD8(+) cells specific for EBV late lytic cycle antigens show poor recognition of target cells compared to immediate early and early antigen-specific CD8(+) cells. This phenomenon is due in part to the early EBV protein BILF1, whose immunosuppressive activity increases with lytic cycle progression. However, published data suggest the existence of a hitherto unidentified immune evasion protein further enhancing protection against late EBV antigen-specific CD8(+) cells. We have now identified the late lytic BDLF3 gene as the missing link accounting for efficient evasion during the late lytic cycle. Interestingly, BDLF3 also contributes to evasion of CD4(+) cell responses to EBV. We report that BDLF3 downregulates expression of surface major histocompatibility complex (MHC) class I and class II molecules in the absence of any effect upon other surface molecules screened, including CD54 (ICAM-1) and CD71 (transferrin receptor). BDLF3 both enhanced internalization of surface MHC molecules and reduced the rate of their appearance at the cell surface. The reduced expression of surface MHC molecules correlated with functional protection against CD8(+) and CD4(+) T cell recognition. The molecular mechanism was identified as BDLF3-induced ubiquitination of MHC molecules and their subsequent downregulation in a proteasome-dependent manner.
Importance: Immune evasion is a necessary feature of viruses that establish lifelong persistent infections in the face of strong immune responses. EBV is an important human pathogen whose immune evasion mechanisms are only partly understood. Of the EBV immune evasion mechanisms identified to date, none could explain why CD8(+) T cell responses to late lytic cycle genes are so infrequent and, when present, recognize lytically infected target cells so poorly relative to CD8(+) T cells specific for early lytic cycle antigens. The present work identifies an additional immune evasion protein, BDLF3, that is expressed late in the lytic cycle and impairs CD8(+) T cell recognition by targeting cell surface MHC class I molecules for ubiquitination and proteasome-dependent downregulation. Interestingly, BDLF3 also targets MHC class II molecules to impair CD4(+) T cell recognition. BDLF3 is therefore a rare example of a viral protein that impairs both the MHC class I and class II antigen-presenting pathways.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
The lytic cycle of Epstein-Barr virus is associated with decreased expression of cell surface major histocompatibility complex class I and class II molecules.J Virol. 2002 Aug;76(16):8179-88. doi: 10.1128/jvi.76.16.8179-8188.2002. J Virol. 2002. PMID: 12134023 Free PMC article.
-
The Epstein-Barr virus-encoded BILF1 protein modulates immune recognition of endogenously processed antigen by targeting major histocompatibility complex class I molecules trafficking on both the exocytic and endocytic pathways.J Virol. 2011 Feb;85(4):1604-14. doi: 10.1128/JVI.01608-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123379 Free PMC article.
-
The Epstein-Barr virus G-protein-coupled receptor contributes to immune evasion by targeting MHC class I molecules for degradation.PLoS Pathog. 2009 Jan;5(1):e1000255. doi: 10.1371/journal.ppat.1000255. Epub 2009 Jan 2. PLoS Pathog. 2009. PMID: 19119421 Free PMC article.
-
Epstein-Barr virus evasion of CD8(+) and CD4(+) T cell immunity via concerted actions of multiple gene products.Semin Cancer Biol. 2008 Dec;18(6):397-408. doi: 10.1016/j.semcancer.2008.10.008. Epub 2008 Oct 25. Semin Cancer Biol. 2008. PMID: 18977445 Review.
-
Immunodominance of lytic cycle antigens in Epstein-Barr virus-specific CD4+ T cell preparations for therapy.PLoS One. 2007 Jul 4;2(7):e583. doi: 10.1371/journal.pone.0000583. PLoS One. 2007. PMID: 17611619 Free PMC article. Review.
Cited by
-
The Obligate Intracellular Bacterium Orientia tsutsugamushi Targets NLRC5 To Modulate the Major Histocompatibility Complex Class I Pathway.Infect Immun. 2019 Feb 21;87(3):e00876-18. doi: 10.1128/IAI.00876-18. Print 2019 Mar. Infect Immun. 2019. PMID: 30559222 Free PMC article.
-
[The diagnostic value of whole blood Epstein-Barr virus DNA load in lymphoproliferative diseases after allogeneic hematopoietic stem cell transplantation].Zhonghua Xue Ye Xue Za Zhi. 2021 Nov 14;42(11):904-910. doi: 10.3760/cma.j.issn.0253-2727.2021.11.004. Zhonghua Xue Ye Xue Za Zhi. 2021. PMID: 35045651 Free PMC article. Chinese.
-
Mechanism and therapeutic implications of pomalidomide-induced immune surface marker upregulation in EBV-positive lymphomas.Sci Rep. 2023 Jul 18;13(1):11596. doi: 10.1038/s41598-023-38156-z. Sci Rep. 2023. PMID: 37463943 Free PMC article.
-
Molecular Properties and Therapeutic Targeting of the EBV-Encoded Receptor BILF1.Cancers (Basel). 2021 Aug 13;13(16):4079. doi: 10.3390/cancers13164079. Cancers (Basel). 2021. PMID: 34439235 Free PMC article. Review.
-
Mimicking the brain: Epstein-Barr virus and foreign agents as drivers of neuroimmune attack in multiple sclerosis.Front Immunol. 2023 Nov 3;14:1304281. doi: 10.3389/fimmu.2023.1304281. eCollection 2023. Front Immunol. 2023. PMID: 38022632 Free PMC article. Review.
References
-
- Rickinson AB, Kieff E. 2007. Epstein-Barr virus, p 2655–2700. In Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE (ed), Fields virology, 5th ed Lippincott Williams & Wilkins, Philadelphia, PA.
-
- Hislop AD, Ressing ME, van Leeuwen D, Pudney VA, Horst D, Koppers-Lalic D, Croft NP, Neefjes JJ, Rickinson AB, Wiertz EJ. 2007. A CD8+ T cell immune evasion protein specific to Epstein-Barr virus and its close relatives in Old World primates. J Exp Med 204:1863–1873. doi:10.1084/jem.20070256. - DOI - PMC - PubMed
-
- Rowe M, Glaunsinger B, van Leeuwen D, Zuo J, Sweetman D, Ganem D, Middeldorp J, Wiertz EJ, Ressing ME. 2007. Host shutoff during productive Epstein-Barr virus infection is mediated by BGLF5 and may contribute to immune evasion. Proc Natl Acad Sci U S A 104:3366–3371. doi:10.1073/pnas.0611128104. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

