NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression
- PMID: 26465118
- PMCID: PMC4744154
- DOI: 10.1111/micc.12251
NG2 Proteoglycan-Dependent Contributions of Pericytes and Macrophages to Brain Tumor Vascularization and Progression
Abstract
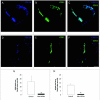
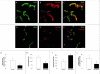
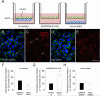
The NG2 proteoglycan promotes tumor growth as a component of both tumor and stromal cells. Using intracranial, NG2-negative B16F10 melanomas, we have investigated the importance of PC and Mac NG2 in brain tumor progression. Reduced melanoma growth in Mac-NG2ko and PC-NG2ko mice demonstrates the importance of NG2 in both stromal compartments. In each genotype, the loss of PC-endothelial cell interaction diminishes the formation of endothelial junctions and assembly of the basal lamina. Tumor vessels in Mac-NG2ko mice have smaller diameters, reduced patency, and increased leakiness compared to PC-NG2ko mice, thus decreasing tumor blood supply and increasing hypoxia. While the reduced PC interaction with endothelial cells in PC-NG2ko mice results from the loss of PC activation of β1 integrin signaling in endothelial cells, reduced PC-endothelial cell interaction in Mac-NG2ko mice results from 90% reduced Mac recruitment. The absence of Mac-derived signals in Mac-NG2ko mice causes the loss of PC association with endothelial cells. Reduced Mac recruitment may be due to diminished activation of integrins in the absence of NG2, causing decreased Mac interaction with endothelial adhesion molecules that are needed for extravasation. These results reflect the complex interplay that occurs between Mac, PC, and endothelial cells during tumor vascularization.
Keywords: macrophage recruitment; neuron-glia antigen 2 proteoglycan; pericyte-endothelial cell interaction; tumor growth; tumor microenvironment; tumor vascularization.
© 2015 John Wiley & Sons Ltd.
Figures

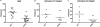
Similar articles
-
NG2 proteoglycan-dependent recruitment of tumor macrophages promotes pericyte-endothelial cell interactions required for brain tumor vascularization.Oncoimmunology. 2015 Jan 22;4(4):e1001204. doi: 10.1080/2162402X.2014.1001204. eCollection 2015 Apr. Oncoimmunology. 2015. PMID: 26137396 Free PMC article.
-
NG2 proteoglycan promotes tumor vascularization via integrin-dependent effects on pericyte function.Angiogenesis. 2014 Jan;17(1):61-76. doi: 10.1007/s10456-013-9378-1. Epub 2013 Aug 8. Angiogenesis. 2014. PMID: 23925489 Free PMC article.
-
Early vascular deficits are correlated with delayed mammary tumorigenesis in the MMTV-PyMT transgenic mouse following genetic ablation of the NG2 proteoglycan.Breast Cancer Res. 2012 Apr 24;14(2):R67. doi: 10.1186/bcr3174. Breast Cancer Res. 2012. PMID: 22531600 Free PMC article.
-
The NG2 Proteoglycan in Pericyte Biology.Adv Exp Med Biol. 2018;1109:5-19. doi: 10.1007/978-3-030-02601-1_2. Adv Exp Med Biol. 2018. PMID: 30523586 Review.
-
NG2 Proteoglycan Enhances Brain Tumor Progression by Promoting Beta-1 Integrin Activation in both Cis and Trans Orientations.Cancers (Basel). 2017 Mar 31;9(4):31. doi: 10.3390/cancers9040031. Cancers (Basel). 2017. PMID: 28362324 Free PMC article. Review.
Cited by
-
Coagulation in Brain Tumors: Biological Basis and Clinical Implications.Front Neurol. 2019 Mar 18;10:181. doi: 10.3389/fneur.2019.00181. eCollection 2019. Front Neurol. 2019. PMID: 30949114 Free PMC article. Review.
-
Nerve-Glial antigen 2: unmasking the enigmatic cellular identity in the central nervous system.Front Immunol. 2024 Jul 29;15:1393842. doi: 10.3389/fimmu.2024.1393842. eCollection 2024. Front Immunol. 2024. PMID: 39136008 Free PMC article. Review.
-
Loss of Endothelium-Derived Wnt5a Is Associated With Reduced Pericyte Recruitment and Small Vessel Loss in Pulmonary Arterial Hypertension.Circulation. 2019 Apr 2;139(14):1710-1724. doi: 10.1161/CIRCULATIONAHA.118.037642. Circulation. 2019. PMID: 30586764 Free PMC article.
-
The Importance of Pericytes in Healing: Wounds and other Pathologies.Int J Mol Sci. 2017 May 24;18(6):1129. doi: 10.3390/ijms18061129. Int J Mol Sci. 2017. PMID: 28538706 Free PMC article. Review.
-
Protein Kinase CK2 Regulates Nerve/Glial Antigen (NG)2-Mediated Angiogenic Activity of Human Pericytes.Cells. 2020 Jun 25;9(6):1546. doi: 10.3390/cells9061546. Cells. 2020. PMID: 32630438 Free PMC article.
References
-
- Allt G, Lawrenson JG. Pericytes: cell biology and pathology. Cells, tissues, organs. 2001;169:1–11. - PubMed
-
- Armulik A, Abramsson A, Betsholtz C. Endothelial/pericyte interactions. Circulation research. 2005;97:512–523. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nature reviews. Cancer. 2003;3:401–410. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

