Enzymatic Dissolution of Biocomposite Solids Consisting of Phosphopeptides to Form Supramolecular Hydrogels
- PMID: 26462722
- PMCID: PMC4743537
- DOI: 10.1002/chem.201504087
Enzymatic Dissolution of Biocomposite Solids Consisting of Phosphopeptides to Form Supramolecular Hydrogels
Abstract
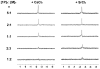
Enzyme-catalyzed dephosphorylation is essential for biomineralization and bone metabolism. Here we report the exploration of using enzymatic reaction to transform biocomposites of phosphopeptides and calcium (or strontium) ions to supramolecular hydrogels as a mimic of enzymatic dissolution of biominerals. (31) P NMR shows that strong affinity between the phosphopeptides and alkaline metal ions (e.g., Ca(2+) or Sr(2+) ) induces the formation of biocomposites as precipitates. Electron microscopy reveals that the enzymatic reaction regulates the morphological transition from particles to nanofibers. Rheology confirms the formation of a rigid hydrogel. As the first example of enzyme-instructed dissolution of a solid to form supramolecular nanofibers/hydrogels, this work provides an approach to generate soft materials with desired properties, expands the application of supramolecular hydrogelators, and offers insights to control the demineralization of calcified soft tissues.
Keywords: bone mineralization; enzyme; phosphopeptide; self-assembly; solid-gel transition.
© 2015 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim.
Figures


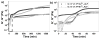

Similar articles
-
Aromatic-aromatic interactions enhance interfiber contacts for enzymatic formation of a spontaneously aligned supramolecular hydrogel.J Am Chem Soc. 2014 Feb 26;136(8):2970-3. doi: 10.1021/ja4127399. Epub 2014 Feb 13. J Am Chem Soc. 2014. PMID: 24512553 Free PMC article.
-
Enzyme-instructed self-assembly of the stereoisomers of pentapeptides to form biocompatible supramolecular hydrogels.J Drug Target. 2020 Aug-Sep;28(7-8):760-765. doi: 10.1080/1061186X.2020.1797048. Epub 2020 Jul 27. J Drug Target. 2020. PMID: 32668995 Free PMC article.
-
Selection of Secondary Structures of Heterotypic Supramolecular Peptide Assemblies by an Enzymatic Reaction.Angew Chem Int Ed Engl. 2018 Sep 3;57(36):11716-11721. doi: 10.1002/anie.201806992. Epub 2018 Aug 6. Angew Chem Int Ed Engl. 2018. PMID: 29971927 Free PMC article.
-
Design Strategies of Stimuli-Responsive Supramolecular Hydrogels Relying on Structural Analyses and Cell-Mimicking Approaches.Acc Chem Res. 2017 Apr 18;50(4):740-750. doi: 10.1021/acs.accounts.7b00070. Epub 2017 Mar 2. Acc Chem Res. 2017. PMID: 28252940 Review.
-
Supramolecular Hydrogels Based on DNA Self-Assembly.Acc Chem Res. 2017 Apr 18;50(4):659-668. doi: 10.1021/acs.accounts.6b00524. Epub 2017 Mar 16. Acc Chem Res. 2017. PMID: 28299927 Review.
Cited by
-
The Enzyme-instructed assembly of the core of yeast prion Sup35 to form supramolecular hydrogels.J Mater Chem B. 2016 Feb 21;4(7):1318-1323. doi: 10.1039/C5TB02346G. Epub 2016 Jan 11. J Mater Chem B. 2016. PMID: 27134750 Free PMC article.
-
Enzymatic Control of the Conformational Landscape of Self-Assembling Peptides.Angew Chem Int Ed Engl. 2018 Aug 27;57(35):11188-11192. doi: 10.1002/anie.201803983. Epub 2018 Jul 25. Angew Chem Int Ed Engl. 2018. PMID: 29969177 Free PMC article.
-
Enzymatic Noncovalent Synthesis.Chem Rev. 2020 Sep 23;120(18):9994-10078. doi: 10.1021/acs.chemrev.0c00306. Epub 2020 Aug 19. Chem Rev. 2020. PMID: 32812754 Free PMC article. Review.
References
-
- Golub EE, Boesze-Battaglia K. Curr Opin Orthop. 2007;18:444–448.
- Addison WN, Azari F, Sorensen ES, Kaartinen MT, McKee MD. J Biol Chem. 2007;282:15872–15883. - PubMed
-
- Sapir-Koren R, Livshits G. IBMS BoneKEy. 2011;8:286–300.
-
- Schneider JP, Pochan DJ, Ozbas B, Rajagopal K, Pakstis L, Kretsinger J. J Am Chem Soc. 2002;124:15030–15037. - PubMed
- Zhang XL, Chu XL, Wang L, Wang HM, Liang GL, Zhang JX, Long JF, Yang ZM. Angew Chem Int Edit. 2012;51:4388–4392. - PubMed
- Micklitsch CM, Knerr PJ, Branco MC, Nagarkar R, Pochan DJ, Schneider JP. Angew Chem Int Edit. 2011;50:1577–1579. - PMC - PubMed
- Cui H, Cheetham AG, Pashuck ET, Stupp SI. J Am Chem Soc. 2014;136:12461–12468. - PMC - PubMed
-
- Schnepp ZAC, Gonzalez-McQuire R, Mann S. Adv Mater. 2006;18:1869–1872.
- Kuang Y, Shi J, Li J, Yuan D, Alberti KA, Xu Q, Xu B. Angew Chem Int Ed. 2014;53:8104–8107. - PMC - PubMed
- Gao Y, Shi J, Yuan D, Xu B. Nat Commun. 2012;3:1033. - PMC - PubMed
- Yang Z, Liang G, Guo Z, Guo Z, Xu B. Angew Chem Int Ed. 2007;46:8216–8219. - PubMed
- Spoerke ED, Anthony SG, Stupp SI. Adv Mater. 2009;21:425–430. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

