α-Arrestins participate in cargo selection for both clathrin-independent and clathrin-mediated endocytosis
- PMID: 26459639
- PMCID: PMC4712785
- DOI: 10.1242/jcs.175372
α-Arrestins participate in cargo selection for both clathrin-independent and clathrin-mediated endocytosis
Abstract
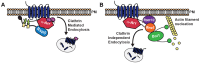
Clathrin-mediated endocytosis (CME) is a well-studied mechanism to internalize plasma membrane proteins; however, to endocytose such cargo, most eukaryotic cells also use alternative clathrin-independent endocytic (CIE) pathways, which are less well characterized. The budding yeast Saccharomyces cerevisiae, a widely used model for studying CME, was recently shown to have a CIE pathway that requires the GTPase Rho1, the formin Bni1, and their regulators. Nevertheless, in both yeast and mammalian cells, the mechanisms underlying cargo selection in CME and CIE are only beginning to be understood. For CME in yeast, particular α-arrestins contribute to recognition of specific cargos and promote their ubiquitylation by recruiting the E3 ubiquitin protein ligase Rsp5. Here, we show that the same α-arrestin-cargo pairs promote internalization through the CIE pathway by interacting with CIE components. Notably, neither expression of Rsp5 nor its binding to α-arrestins is required for CIE. Thus, α-arrestins are important for cargo selection in both the CME and CIE pathways, but function by distinct mechanisms in each pathway.
Keywords: Internalization; Plasma membrane; Protein trafficking; Ubiquitin ligase; Yeast.
© 2015. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures


Similar articles
-
Internalization of Heterologous Sugar Transporters by Endogenous α-Arrestins in the Yeast Saccharomyces cerevisiae.Appl Environ Microbiol. 2016 Nov 21;82(24):7074-7085. doi: 10.1128/AEM.02148-16. Print 2016 Dec 15. Appl Environ Microbiol. 2016. PMID: 27694235 Free PMC article.
-
Differential Phosphorylation Provides a Switch to Control How α-Arrestin Rod1 Down-regulates Mating Pheromone Response in Saccharomyces cerevisiae.Genetics. 2016 May;203(1):299-317. doi: 10.1534/genetics.115.186122. Epub 2016 Feb 26. Genetics. 2016. PMID: 26920760 Free PMC article.
-
Syp1 regulates the clathrin-mediated and clathrin-independent endocytosis of multiple cargo proteins through a novel sorting motif.Mol Biol Cell. 2017 Sep 1;28(18):2434-2448. doi: 10.1091/mbc.E15-10-0731. Epub 2017 Jul 12. Mol Biol Cell. 2017. PMID: 28701344 Free PMC article.
-
The α-arrestin family of ubiquitin ligase adaptors links metabolism with selective endocytosis.Biol Cell. 2021 Apr;113(4):183-219. doi: 10.1111/boc.202000137. Epub 2021 Feb 1. Biol Cell. 2021. PMID: 33314196 Review.
-
Mechanisms of Carrier Formation during Clathrin-Independent Endocytosis.Trends Cell Biol. 2018 Mar;28(3):188-200. doi: 10.1016/j.tcb.2017.11.004. Epub 2017 Dec 11. Trends Cell Biol. 2018. PMID: 29241687 Review.
Cited by
-
Tracking yeast pheromone receptor Ste2 endocytosis using fluorogen-activating protein tagging.Mol Biol Cell. 2018 Nov 1;29(22):2720-2736. doi: 10.1091/mbc.E18-07-0424. Epub 2018 Sep 12. Mol Biol Cell. 2018. PMID: 30207829 Free PMC article.
-
Applications of pHluorin for Quantitative, Kinetic and High-throughput Analysis of Endocytosis in Budding Yeast.J Vis Exp. 2016 Oct 23;(116):54587. doi: 10.3791/54587. J Vis Exp. 2016. PMID: 27805610 Free PMC article.
-
AMPK-Mediated Regulation of Alpha-Arrestins and Protein Trafficking.Int J Mol Sci. 2019 Jan 25;20(3):515. doi: 10.3390/ijms20030515. Int J Mol Sci. 2019. PMID: 30691068 Free PMC article. Review.
-
Actin- and microtubule-based motors contribute to clathrin-independent endocytosis in yeast.Mol Biol Cell. 2023 Nov 1;34(12):ar117. doi: 10.1091/mbc.E23-05-0164. Epub 2023 Aug 30. Mol Biol Cell. 2023. PMID: 37647159 Free PMC article.
-
Select α-arrestins control cell-surface abundance of the mammalian Kir2.1 potassium channel in a yeast model.J Biol Chem. 2018 Jul 13;293(28):11006-11021. doi: 10.1074/jbc.RA117.001293. Epub 2018 May 21. J Biol Chem. 2018. PMID: 29784874 Free PMC article.
References
-
- Aguilar R. C., Longhi S. A., Shaw J. D., Yeh L.-Y., Kim S., Schön A., Freire E., Hsu A., McCormick W. K., Watson H. A. et al. (2006). Epsin N-terminal homology domains perform an essential function regulating Cdc42 through binding Cdc42 GTPase-activating proteins. Proc. Natl. Acad. Sci. USA 103, 4116-4121. 10.1073/pnas.0510513103 - DOI - PMC - PubMed
-
- Alvaro C. G., O'Donnell A. F., Prosser D. C., Augustine A. A., Goldman A., Brodsky J. L., Cyert M. S., Wendland B. and Thorner J. (2014). Specific alpha-arrestins negatively regulate saccharomyces cerevisiae pheromone response by down-modulating the G-protein-coupled receptor Ste2. Mol. Cell. Biol. 34, 2660-2681. 10.1128/MCB.00230-14 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

