The endocytic recycling regulatory protein EHD1 Is required for ocular lens development
- PMID: 26455409
- PMCID: PMC4688215
- DOI: 10.1016/j.ydbio.2015.10.005
The endocytic recycling regulatory protein EHD1 Is required for ocular lens development
Abstract
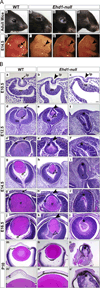
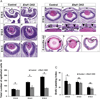
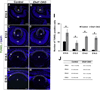
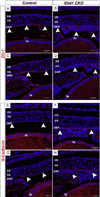
The C-terminal Eps15 homology domain-containing (EHD) proteins play a key role in endocytic recycling, a fundamental cellular process that ensures the return of endocytosed membrane components and receptors back to the cell surface. To define the in vivo biological functions of EHD1, we have generated Ehd1 knockout mice and previously reported a requirement of EHD1 for spermatogenesis. Here, we show that approximately 56% of the Ehd1-null mice displayed gross ocular abnormalities, including anophthalmia, aphakia, microphthalmia and congenital cataracts. Histological characterization of ocular abnormalities showed pleiotropic defects that include a smaller or absent lens, persistence of lens stalk and hyaloid vasculature, and deformed optic cups. To test whether these profound ocular defects resulted from the loss of EHD1 in the lens or in non-lenticular tissues, we deleted the Ehd1 gene selectively in the presumptive lens ectoderm using Le-Cre. Conditional Ehd1 deletion in the lens resulted in developmental defects that included thin epithelial layers, small lenses and absence of corneal endothelium. Ehd1 deletion in the lens also resulted in reduced lens epithelial proliferation, survival and expression of junctional proteins E-cadherin and ZO-1. Finally, Le-Cre-mediated deletion of Ehd1 in the lens led to defects in corneal endothelial differentiation. Taken together, these data reveal a unique role for EHD1 in early lens development and suggest a previously unknown link between the endocytic recycling pathway and regulation of key developmental processes including proliferation, differentiation and morphogenesis.
Keywords: Apoptosis; Corneal endothelium; EHD1; Endocytic recycling; Lens development; Lens epithelium; Polarity; Proliferation.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Rap1 GTPase is required for mouse lens epithelial maintenance and morphogenesis.Dev Biol. 2015 Oct 1;406(1):74-91. doi: 10.1016/j.ydbio.2015.06.022. Epub 2015 Jul 23. Dev Biol. 2015. PMID: 26212757 Free PMC article.
-
Co-operative roles for E-cadherin and N-cadherin during lens vesicle separation and lens epithelial cell survival.Dev Biol. 2009 Feb 15;326(2):403-17. doi: 10.1016/j.ydbio.2008.10.011. Epub 2008 Nov 1. Dev Biol. 2009. PMID: 18996109 Free PMC article.
-
Ankyrin-G regulated epithelial phenotype is required for mouse lens morphogenesis and growth.Dev Biol. 2019 Feb 1;446(1):119-131. doi: 10.1016/j.ydbio.2018.12.016. Epub 2018 Dec 15. Dev Biol. 2019. PMID: 30562487 Free PMC article.
-
Apoptosis: its functions and control in the ocular lens.Curr Mol Med. 2010 Dec;10(9):864-75. doi: 10.2174/156652410793937741. Curr Mol Med. 2010. PMID: 21091420 Review.
-
Systems biology of lens development: A paradigm for disease gene discovery in the eye.Exp Eye Res. 2017 Mar;156:22-33. doi: 10.1016/j.exer.2016.03.010. Epub 2016 Mar 16. Exp Eye Res. 2017. PMID: 26992779 Free PMC article. Review.
Cited by
-
Role of the EHD Family of Endocytic Recycling Regulators for TCR Recycling and T Cell Function.J Immunol. 2018 Jan 15;200(2):483-499. doi: 10.4049/jimmunol.1601793. Epub 2017 Dec 6. J Immunol. 2018. PMID: 29212907 Free PMC article.
-
A missense mutation in Ehd1 associated with defective spermatogenesis and male infertility.Front Cell Dev Biol. 2023 Oct 12;11:1240558. doi: 10.3389/fcell.2023.1240558. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 37900275 Free PMC article.
-
WAVE facilitates polarized E-cadherin transport.Mol Biol Cell. 2023 May 1;34(5):ar44. doi: 10.1091/mbc.E22-08-0322. Epub 2023 Mar 22. Mol Biol Cell. 2023. PMID: 36947190 Free PMC article.
-
Hedgehog Signal and Genetic Disorders.Front Genet. 2019 Nov 8;10:1103. doi: 10.3389/fgene.2019.01103. eCollection 2019. Front Genet. 2019. PMID: 31781166 Free PMC article. Review.
-
Apoptosis generates mechanical forces that close the lens vesicle in the chick embryo.Phys Biol. 2018 Feb 8;15(2):025001. doi: 10.1088/1478-3975/aa8d0e. Phys Biol. 2018. PMID: 28914615 Free PMC article.
References
-
- Ashery-Padan R, Gruss P. Pax6 lights-up the way for eye development. Curr. Opin. Cell Biol. 2001;13:706–714. - PubMed
-
- Bassnett S, Kuszak JR, Reinisch L, Brown HG, Beebe DC. Intercellular communication between epithelial and fiber cells of the eye lens. J. Cell. Sci. 1994;107(Pt 4):799–811. - PubMed
-
- Beebe DC, Coats JM. The lens organizes the anterior segment: Specification of neural crest cell differentiation in the avian eye. Dev. Biol. 2000;220:424–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 CA087986/CA/NCI NIH HHS/United States
- CA105489/CA/NCI NIH HHS/United States
- R01 CA096844/CA/NCI NIH HHS/United States
- CA96844/CA/NCI NIH HHS/United States
- CA116552/CA/NCI NIH HHS/United States
- EY017610/EY/NEI NIH HHS/United States
- R01 CA116552/CA/NCI NIH HHS/United States
- R01 CA144027/CA/NCI NIH HHS/United States
- R01 CA105489/CA/NCI NIH HHS/United States
- R01 EY017610/EY/NEI NIH HHS/United States
- CA99163/CA/NCI NIH HHS/United States
- R01 CA099163/CA/NCI NIH HHS/United States
- CA144027/CA/NCI NIH HHS/United States
- CA87986/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

