A novel siderophore system is essential for the growth of Pseudomonas aeruginosa in airway mucus
- PMID: 26446565
- PMCID: PMC4597187
- DOI: 10.1038/srep14644
A novel siderophore system is essential for the growth of Pseudomonas aeruginosa in airway mucus
Abstract
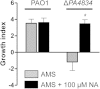
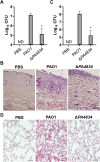
Pseudomonas aeruginosa establishes airway infections in Cystic Fibrosis patients. Here, we investigate the molecular interactions between P. aeruginosa and airway mucus secretions (AMS) derived from the primary cultures of normal human tracheal epithelial (NHTE) cells. PAO1, a prototype strain of P. aeruginosa, was capable of proliferating during incubation with AMS, while all other tested bacterial species perished. A PAO1 mutant lacking PA4834 gene became susceptible to AMS treatment. The ΔPA4834 mutant was grown in AMS supplemented with 100 μM ferric iron, suggesting that the PA4834 gene product is involved in iron metabolism. Consistently, intracellular iron content was decreased in the mutant, but not in PAO1 after the AMS treatment. Importantly, a PAO1 mutant unable to produce both pyoverdine and pyochelin remained viable, suggesting that these two major siderophore molecules are dispensable for maintaining viability during incubation with AMS. The ΔPA4834 mutant was regrown in AMS amended with 100 μM nicotianamine, a phytosiderophore whose production is predicted to be mediated by the PA4836 gene. Infectivity of the ΔPA4834 mutant was also significantly compromised in vivo. Together, our results identify a genetic element encoding a novel iron acquisition system that plays a previously undiscovered role in P. aeruginosa airway infection.
Figures
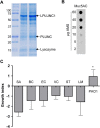
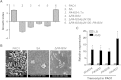
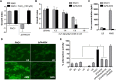
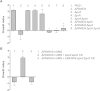
Similar articles
-
Ferric Uptake Regulator Fur Is Conditionally Essential in Pseudomonas aeruginosa.J Bacteriol. 2017 Oct 17;199(22):e00472-17. doi: 10.1128/JB.00472-17. Print 2017 Nov 15. J Bacteriol. 2017. PMID: 28847923 Free PMC article.
-
The Pseudomonas aeruginosa pirA gene encodes a second receptor for ferrienterobactin and synthetic catecholate analogues.FEMS Microbiol Lett. 2005 May 15;246(2):167-74. doi: 10.1016/j.femsle.2005.04.010. FEMS Microbiol Lett. 2005. PMID: 15899402
-
Role of Iron Uptake Systems in Pseudomonas aeruginosa Virulence and Airway Infection.Infect Immun. 2016 Jul 21;84(8):2324-2335. doi: 10.1128/IAI.00098-16. Print 2016 Aug. Infect Immun. 2016. PMID: 27271740 Free PMC article.
-
Roles of Pseudomonas aeruginosa siderophores in interaction with prokaryotic and eukaryotic organisms.Res Microbiol. 2024 Sep-Oct;175(7):104211. doi: 10.1016/j.resmic.2024.104211. Epub 2024 May 9. Res Microbiol. 2024. PMID: 38734157 Review.
-
Aspergillus-Pseudomonas interaction, relevant to competition in airways.Med Mycol. 2019 Apr 1;57(Supplement_2):S228-S232. doi: 10.1093/mmy/myy087. Med Mycol. 2019. PMID: 30816973 Review.
Cited by
-
A Review of Pseudomonas aeruginosa Metallophores: Pyoverdine, Pyochelin and Pseudopaline.Biology (Basel). 2022 Nov 25;11(12):1711. doi: 10.3390/biology11121711. Biology (Basel). 2022. PMID: 36552220 Free PMC article. Review.
-
Chalkophores.Annu Rev Biochem. 2018 Jun 20;87:645-676. doi: 10.1146/annurev-biochem-062917-012300. Epub 2018 Apr 18. Annu Rev Biochem. 2018. PMID: 29668305 Free PMC article. Review.
-
Investigation of Zur-regulated metal transport systems reveals an unexpected role of pyochelin in zinc homeostasis.mBio. 2024 Oct 16;15(10):e0239524. doi: 10.1128/mbio.02395-24. Epub 2024 Sep 24. mBio. 2024. PMID: 39315802 Free PMC article.
-
Unraveling the genomic secrets of Tritonibacter mobilis AK171: a plant growth-promoting bacterium isolated from Avicennia marina.BMC Genomics. 2024 Jul 5;25(1):672. doi: 10.1186/s12864-024-10555-0. BMC Genomics. 2024. PMID: 38969999 Free PMC article.
-
The Metallophore Staphylopine Enables Staphylococcus aureus To Compete with the Host for Zinc and Overcome Nutritional Immunity.mBio. 2017 Oct 31;8(5):e01281-17. doi: 10.1128/mBio.01281-17. mBio. 2017. PMID: 29089427 Free PMC article.
References
-
- Smith J. J., Travis S. M., Greenberg E. P. & Welsh M. J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell 85, 229–236 (1996). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

