Importance of the Side Chain at Position 296 of Antibody Fc in Interactions with FcγRIIIa and Other Fcγ Receptors
- PMID: 26444434
- PMCID: PMC4596520
- DOI: 10.1371/journal.pone.0140120
Importance of the Side Chain at Position 296 of Antibody Fc in Interactions with FcγRIIIa and Other Fcγ Receptors
Abstract
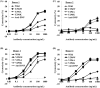
Antibody-dependent cellular cytotoxicity (ADCC) is an important effector function determining the clinical efficacy of therapeutic antibodies. Core fucose removal from N-glycans on the Fc portion of immunoglobulin G (IgG) improves the binding affinity for Fcγ receptor IIIa (FcγRIIIa) and dramatically enhances ADCC. Our previous structural analyses revealed that Tyr-296 of IgG1-Fc plays a critical role in the interaction with FcγRIIIa, particularly in the enhanced FcγRIIIa binding of nonfucosylated IgG1. However, the importance of the Tyr-296 residue in the antibody in the interaction with various Fcγ receptors has not yet been elucidated. To further clarify the biological importance of this residue, we established comprehensive Tyr-296 mutants as fucosylated and nonfucosylated anti-CD20 IgG1s rituximab variants and examined their binding to recombinant soluble human Fcγ receptors: shFcγRI, shFcγRIIa, shFcγRIIIa, and shFcγRIIIb. Some of the mutations affected the binding of antibody to not only shFcγRIIIa but also shFcγRIIa and shFcγRIIIb, suggesting that the Tyr-296 residue in the antibody was also involved in interactions with FcγRIIa and FcγRIIIb. For FcγRIIIa binding, almost all Tyr-296 variants showed lower binding affinities than the wild-type antibody, irrespective of their core fucosylation, particularly in Y296K and Y296P. Notably, only the Y296W mutant showed improved binding to FcγRIIIa. The 3.00 Å-resolution crystal structure of the nonfucosylated Y296W mutant in complex with shFcγRIIIa harboring two N-glycans revealed that the Tyr-to-Trp substitution increased the number of potential contact atoms in the complex, thus improving the binding of the antibody to shFcγRIIIa. The nonfucosylated Y296W mutant retained high ADCC activity, relative to the nonfucosylated wild-type IgG1, and showed greater binding affinity for FcγRIIa. Our data may improve our understanding of the biological importance of human IgG1-Fc Tyr-296 in interactions with various Fcγ receptors, and have applications in the modulation of the IgG1-Fc function of therapeutic antibodies.
Conflict of interest statement
Figures


Similar articles
-
Nonfucosylated therapeutic IgG1 antibody can evade the inhibitory effect of serum immunoglobulin G on antibody-dependent cellular cytotoxicity through its high binding to FcgammaRIIIa.Clin Cancer Res. 2006 May 1;12(9):2879-87. doi: 10.1158/1078-0432.CCR-05-2619. Clin Cancer Res. 2006. PMID: 16675584
-
Structural basis for improved efficacy of therapeutic antibodies on defucosylation of their Fc glycans.Genes Cells. 2011 Nov;16(11):1071-80. doi: 10.1111/j.1365-2443.2011.01552.x. Genes Cells. 2011. PMID: 22023369 Free PMC article.
-
The N-linked oligosaccharide at Fc gamma RIIIa Asn-45: an inhibitory element for high Fc gamma RIIIa binding affinity to IgG glycoforms lacking core fucosylation.Glycobiology. 2009 Feb;19(2):126-34. doi: 10.1093/glycob/cwn110. Epub 2008 Oct 24. Glycobiology. 2009. PMID: 18952826 Free PMC article.
-
The "less-is-more" in therapeutic antibodies: Afucosylated anti-cancer antibodies with enhanced antibody-dependent cellular cytotoxicity.MAbs. 2018 Jul;10(5):693-711. doi: 10.1080/19420862.2018.1466767. MAbs. 2018. PMID: 29733746 Free PMC article. Review.
-
New anti-CD20 monoclonal antibodies for the treatment of B-cell lymphoid malignancies.BioDrugs. 2011 Feb 1;25(1):13-25. doi: 10.2165/11539590-000000000-00000. BioDrugs. 2011. PMID: 21090841 Review.
Cited by
-
Dynamic Views of the Fc Region of Immunoglobulin G Provided by Experimental and Computational Observations.Antibodies (Basel). 2019 Jul 1;8(3):39. doi: 10.3390/antib8030039. Antibodies (Basel). 2019. PMID: 31544845 Free PMC article.
-
Profiling the Biophysical Developability Properties of Common IgG1 Fc Effector Silencing Variants.Antibodies (Basel). 2023 Aug 22;12(3):54. doi: 10.3390/antib12030054. Antibodies (Basel). 2023. PMID: 37753968 Free PMC article.
-
The Fab portion of immunoglobulin G contributes to its binding to Fcγ receptor III.Sci Rep. 2019 Aug 16;9(1):11957. doi: 10.1038/s41598-019-48323-w. Sci Rep. 2019. PMID: 31420591 Free PMC article.
-
Conformational effects of N-glycan core fucosylation of immunoglobulin G Fc region on its interaction with Fcγ receptor IIIa.Sci Rep. 2017 Oct 23;7(1):13780. doi: 10.1038/s41598-017-13845-8. Sci Rep. 2017. PMID: 29062024 Free PMC article.
-
Biophysical characterization of dynamic structures of immunoglobulin G.Biophys Rev. 2020 Jun;12(3):637-645. doi: 10.1007/s12551-020-00698-1. Epub 2020 May 15. Biophys Rev. 2020. PMID: 32410186 Free PMC article. Review.
References
-
- Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J Clin Oncol. 2003;21(21):3940–7. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

