Inflammation-associated extracellular β-glucuronidase alters cellular responses to the chemical carcinogen benzo[a]pyrene
- PMID: 26438400
- PMCID: PMC4982897
- DOI: 10.1007/s00204-015-1593-7
Inflammation-associated extracellular β-glucuronidase alters cellular responses to the chemical carcinogen benzo[a]pyrene
Abstract
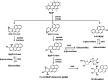
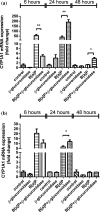
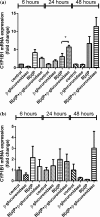
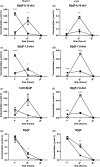
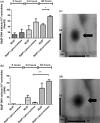
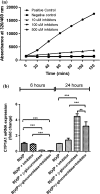
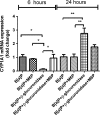
Neutrophils infiltrate tissues during inflammation, and when activated, they release β-glucuronidase. Since inflammation is associated with carcinogenesis, we investigated how extracellular β-glucuronidase changed the in vitro cellular response to the chemical carcinogen benzo(a)pyrene (B[a]P). For this we exposed human liver (HepG2) and lung (A549) cells to B[a]P in the presence or absence of β-glucuronidase. β-Glucuronidase reduced B[a]P-induced expression of CYP1A1 and CYP1B1 at 6 h after exposure, which did not depend on β-glucuronidase activity, because the inhibitor D-saccharic acid 1,4-lactone monohydrate did not antagonize the effect of β-glucuronidase. On the other hand, the inhibitory effect of β-glucuronidase on CYP expression was dependent on signalling via the insulin-like growth factor receptor (IGF2R, a known receptor for β-glucuronidase), because co-incubation with the IGF2R inhibitor mannose-6-phosphate completely abolished the effect of β-glucuronidase. Extracellular β-glucuronidase also reduced the formation of several B[a]P metabolites and B[a]P-DNA adducts. Interestingly, at 24 h of exposure, β-glucuronidase significantly enhanced CYP expression, probably because β-glucuronidase de-glucuronidated B[a]P metabolites, which continued to trigger the aryl hydrocarbon receptor (Ah receptor) and induced expression of CYP1A1 (in both cell lines) and CYP1B1 (in A549 only). Consequently, significantly higher concentrations of B[a]P metabolites and DNA adducts were found in β-glucuronidase-treated cells at 24 h. DNA adduct levels peaked at 48 h in cells that were exposed to B[a]P and treated with β-glucuronidase. Overall, these data show that β-glucuronidase alters the cellular response to B[a]P and ultimately enhances B[a]P-induced DNA adduct levels.
Keywords: Benzo[a]pyrene; Carcinogen metabolism; Cytochrome P450 1A1; DNA adducts; IGF2R; Inflammation; β-Glucuronidase.
Conflict of interest statement
The authors declare that there are no conflicts of interest in this study.
Figures


Similar articles
-
Tissue specific induction of cytochrome P450 (CYP) 1A1 and 1B1 in rat liver and lung following in vitro (tissue slice) and in vivo exposure to benzo(a)pyrene.Toxicol In Vitro. 2006 Jun;20(4):426-38. doi: 10.1016/j.tiv.2005.08.015. Epub 2005 Sep 28. Toxicol In Vitro. 2006. PMID: 16198082
-
Carcinogenic polycyclic aromatic hydrocarbons induce CYP1A1 in human cells via a p53-dependent mechanism.Arch Toxicol. 2016 Feb;90(2):291-304. doi: 10.1007/s00204-014-1409-1. Epub 2014 Nov 15. Arch Toxicol. 2016. PMID: 25398514 Free PMC article.
-
E-cigarette Aerosol Condensate Enhances Metabolism of Benzo(a)pyrene to Genotoxic Products, and Induces CYP1A1 and CYP1B1, Likely by Activation of the Aryl Hydrocarbon Receptor.Int J Environ Res Public Health. 2019 Jul 11;16(14):2468. doi: 10.3390/ijerph16142468. Int J Environ Res Public Health. 2019. PMID: 31373329 Free PMC article.
-
Differential response to benzo[A]pyrene in human lung adenocarcinoma cell lines: the absence of aryl hydrocarbon receptor activation.Life Sci. 1999;65(13):1339-49. doi: 10.1016/s0024-3205(99)00373-2. Life Sci. 1999. PMID: 10503953
-
CYP1A1 and GSTM1 genotypes affect benzo[a]pyrene DNA adducts in smokers' lung: comparison with aromatic/hydrophobic adduct formation.Carcinogenesis. 2002 Dec;23(12):1969-77. doi: 10.1093/carcin/23.12.1969. Carcinogenesis. 2002. PMID: 12507920 Review.
Cited by
-
The Effect of Probiotic Supplementation on the Gut-Brain Axis in Psychiatric Patients.Curr Issues Mol Biol. 2023 May 6;45(5):4080-4099. doi: 10.3390/cimb45050260. Curr Issues Mol Biol. 2023. PMID: 37232729 Free PMC article. Review.
-
Molecular Mechanisms Contributing Bacterial Infections to the Incidence of Various Types of Cancer.Mediators Inflamm. 2020 Jul 8;2020:4070419. doi: 10.1155/2020/4070419. eCollection 2020. Mediators Inflamm. 2020. PMID: 32724295 Free PMC article. Review.
-
Genotoxicity of fine and coarse fraction ambient particulate matter in immortalised normal (TT1) and cancer-derived (A549) alveolar epithelial cells.Environ Mol Mutagen. 2018 May;59(4):290-301. doi: 10.1002/em.22166. Epub 2018 Jan 25. Environ Mol Mutagen. 2018. PMID: 29368350 Free PMC article.
-
Acidic cellular microenvironment modifies carcinogen-induced DNA damage and repair.Arch Toxicol. 2017 Jun;91(6):2425-2441. doi: 10.1007/s00204-016-1907-4. Epub 2016 Dec 22. Arch Toxicol. 2017. PMID: 28005143 Free PMC article.
-
Global DNA methylation changes in rock pigeon (Columba livia) as a sentinel species due to polycyclic aromatic hydrocarbons exposure in Tehran (Iran) as a megacity.Environ Sci Pollut Res Int. 2019 Sep;26(25):26090-26101. doi: 10.1007/s11356-019-05642-9. Epub 2019 Jul 6. Environ Sci Pollut Res Int. 2019. PMID: 31280440
References
-
- Basinska A, Florianczyk B. Beta-glucuronidase in physiology and disease. Ann Univ Mariae Curie Sklodowska Med. 2003;58:386–389. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

