Microbial Nucleic Acid Sensing in Oral and Systemic Diseases
- PMID: 26438211
- PMCID: PMC4700663
- DOI: 10.1177/0022034515609062
Microbial Nucleic Acid Sensing in Oral and Systemic Diseases
Abstract
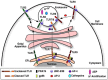
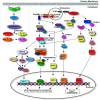
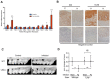
One challenge in studying chronic infectious and inflammatory disorders is understanding how host pattern recognition receptors (PRRs), specifically toll-like receptors (TLRs), sense and respond to pathogen- or damage-associated molecular patterns, their communication with each other and different components of the immune system, and their role in propagating inflammatory stages of disease. The discovery of innate immune activation through nucleic acid recognition by intracellular PRRs such as endosomal TLRs (TLR3, TLR7, TLR8, and TLR9) and cytoplasmic proteins (absent in melanoma 2 and DNA-dependent activator of interferon regulatory factor) opened a new paradigm: Nucleic acid sensing is now implicated in multiple immune and inflammatory conditions (e.g., atherosclerosis, cancer), viral (e.g., human papillomavirus, herpes virus) and bacterial (e.g., Helicobacter pylori, pneumonia) diseases, and autoimmune disorders (e.g., systemic lupus erythematosus, rheumatoid arthritis). Clinical investigations reveal the overexpression of specific nucleic acid sensors in diseased tissues. In vivo animal models show enhanced disease progression associated with receptor activation. The involvement of nucleic acid sensors in various systemic conditions is further supported by studies reporting receptor knockout mice being either protected from or prone to disease. TLR9-mediated inflammation is also implicated in periodontal diseases. Considering that persistent inflammation in the oral cavity is associated with systemic diseases and that oral microbial DNA is isolated at distal sites, nucleic acid sensing may potentially be a link between oral and systemic diseases. In this review, we discuss recent advances in how intracellular PRRs respond to microbial nucleic acids and emerging views on the role of nucleic acid sensors in various systemic diseases. We also highlight new information on the role of intracellular PRRs in the pathogenesis of oral diseases including periodontitis and oral cavity cancer, which might offer future possibilities for disease prevention and therapy.
Keywords: AIM2; DAI; infection; inflammation; periodontal disease; toll-like receptor.
© International & American Associations for Dental Research 2015.
Conflict of interest statement
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
Figures
Similar articles
-
Antiviral signaling through pattern recognition receptors.J Biochem. 2007 Feb;141(2):137-45. doi: 10.1093/jb/mvm032. Epub 2006 Dec 26. J Biochem. 2007. PMID: 17190786 Review.
-
Recognition of bacterial infection by innate immune sensors.Crit Rev Microbiol. 2013 Aug;39(3):229-46. doi: 10.3109/1040841X.2012.706249. Epub 2012 Aug 6. Crit Rev Microbiol. 2013. PMID: 22866947 Review.
-
Signaling Through Nucleic Acid Sensors and Their Roles in Inflammatory Diseases.Front Immunol. 2021 Jan 28;11:625833. doi: 10.3389/fimmu.2020.625833. eCollection 2020. Front Immunol. 2021. PMID: 33633744 Free PMC article. Review.
-
Mechanisms and pathways of innate immune activation and regulation in health and cancer.Hum Vaccin Immunother. 2014;10(11):3270-85. doi: 10.4161/21645515.2014.979640. Hum Vaccin Immunother. 2014. PMID: 25625930 Free PMC article. Review.
-
Essential role of high-mobility group box proteins in nucleic acid-mediated innate immune responses.J Intern Med. 2011 Oct;270(4):301-8. doi: 10.1111/j.1365-2796.2011.02433.x. J Intern Med. 2011. PMID: 21793952
Cited by
-
Estimation of Toll-like receptor 9 in gingival tissues of patients with chronic periodontitis with or without hyperlipidemia and its association with the presence of Porphyromonas gingivalis.J Indian Soc Periodontol. 2018 Jul-Aug;22(4):298-303. doi: 10.4103/jisp.jisp_124_18. J Indian Soc Periodontol. 2018. PMID: 30131620 Free PMC article.
-
A20 Orchestrates Inflammatory Response in the Oral Mucosa through Restraining NF-κB Activity.J Immunol. 2019 Apr 1;202(7):2044-2056. doi: 10.4049/jimmunol.1801286. Epub 2019 Feb 13. J Immunol. 2019. PMID: 30760622 Free PMC article.
-
TLR9 Mediates Periodontal Aging by Fostering Senescence and Inflammaging.J Dent Res. 2022 Dec;101(13):1628-1636. doi: 10.1177/00220345221110108. Epub 2022 Aug 2. J Dent Res. 2022. PMID: 35918888 Free PMC article.
-
The tRNAVal half: A strong endogenous Toll-like receptor 7 ligand with a 5'-terminal universal sequence signature.Proc Natl Acad Sci U S A. 2024 May 7;121(19):e2319569121. doi: 10.1073/pnas.2319569121. Epub 2024 Apr 29. Proc Natl Acad Sci U S A. 2024. PMID: 38683985 Free PMC article.
-
Quercetin Preserves Oral Cavity Health by Mitigating Inflammation and Microbial Dysbiosis.Front Immunol. 2021 Nov 26;12:774273. doi: 10.3389/fimmu.2021.774273. eCollection 2021. Front Immunol. 2021. PMID: 34899728 Free PMC article.
References
-
- Ahn MY, Kwon SM, Cheong HH, Park JH, Lee J, Min SK, Ahn SG, Yoon JH. 2012. Toll-like receptor 7 agonist, imiquimod, inhibits oral squamous carcinoma cells through apoptosis and necrosis. J Oral Pathol Med. 41(7):540–546. - PubMed
-
- Armingohar Z, Jorgensen JJ, Kristoffersen AK, Abesha-Belay E, Olsen I. 2014. Bacteria and bacterial DNA in atherosclerotic plaque and aneurysmal wall biopsies from patients with and without periodontitis. J Oral Microbiol. [epub ahead of print 2014. May 15]. doi:10.3402/jom.v6.23408. - DOI - PMC - PubMed
-
- Bhan U, Lukacs NW, Osterholzer JJ, Newstead MW, Zeng X, Moore TA, McMillan TR, Krieg AM, Akira S, Standiford TJ. 2007. TLR9 is required for protective innate immunity in gram-negative bacterial pneumonia: role of dendritic cells. J Immunol. 179(6):3937–3946. - PubMed
-
- Bostanci N, Meier A, Guggenheim B, Belibasakis GN. 2011. Regulation of NLRP3 and AIM2 inflammasome gene expression levels in gingival fibroblasts by oral biofilms. Cell Immunol. 270(1):88–93. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

