Ca2+ influx through L-type Ca2+ channels and Ca2+-induced Ca2+ release regulate cAMP accumulation and Epac1-dependent ERK 1/2 activation in INS-1 cells
- PMID: 26435461
- PMCID: PMC4684454
- DOI: 10.1016/j.mce.2015.09.034
Ca2+ influx through L-type Ca2+ channels and Ca2+-induced Ca2+ release regulate cAMP accumulation and Epac1-dependent ERK 1/2 activation in INS-1 cells
Abstract
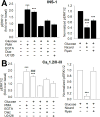
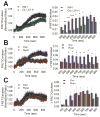
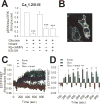
We previously reported that INS-1 cells expressing the intracellular II-III loop of the L-type Ca(2+) channel Cav1.2 (Cav1.2/II-III cells) are deficient in Ca(2+)-induced Ca(2+) release (CICR). Here we show that glucose-stimulated ERK 1/2 phosphorylation (GSEP) is slowed and reduced in Cav1.2/II-III cells compared to INS-1 cells. This parallels a decrease in glucose-stimulated cAMP accumulation (GS-cAMP) in Cav1.2/II-III cells. Influx of Ca(2+) via L-type Ca(2+) channels and CICR play roles in both GSEP and GS-cAMP in INS-1 cells since both are inhibited by nicardipine or ryanodine. Further, the Epac1-selective inhibitor CE3F4 abolishes glucose-stimulated ERK activation in INS-1 cells, as measured using the FRET-based sensor EKAR. The non-selective Epac antagonist ESI-09 but not the Epac2-selective antagonist ESI-05 nor the PKA antagonist Rp-cAMPs inhibits GSEP in both INS-1 and Cav1.2/II-III cells. We conclude that L-type Ca(2+) channel-dependent cAMP accumulation, that's amplified by CICR, activates Epac1 and drives GSEP in INS-1 cells.
Keywords: Ca(2+)-induced Ca(2+) release; Ca(v)1.2; Exchange protein directly activated by cAMP; Extracellular signal regulated kinase 1/2; L-type Ca(2+) channel; Pancreatic beta-cell; cAMP.
Copyright © 2015 Elsevier Ireland Ltd. All rights reserved.
Figures



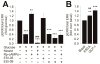

Similar articles
-
cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic beta-cells.J Physiol. 2001 Oct 15;536(Pt 2):375-85. doi: 10.1111/j.1469-7793.2001.0375c.xd. J Physiol. 2001. PMID: 11600673 Free PMC article.
-
Differential modulation of Cav1.2 and Cav1.3-mediated glucose-stimulated insulin secretion by cAMP in INS-1 cells: distinct roles for exchange protein directly activated by cAMP 2 (Epac2) and protein kinase A.J Pharmacol Exp Ther. 2006 Jul;318(1):152-60. doi: 10.1124/jpet.105.097477. Epub 2006 Mar 24. J Pharmacol Exp Ther. 2006. PMID: 16565168
-
Uncoupling of Cav1.2 from Ca(2+)-induced Ca(2+) release and SK channel regulation in pancreatic β-cells.Mol Endocrinol. 2014 Apr;28(4):458-76. doi: 10.1210/me.2013-1094. Epub 2014 Feb 7. Mol Endocrinol. 2014. PMID: 24506535 Free PMC article.
-
Epac in cardiac calcium signaling.J Mol Cell Cardiol. 2013 May;58:162-71. doi: 10.1016/j.yjmcc.2012.11.021. Epub 2012 Dec 7. J Mol Cell Cardiol. 2013. PMID: 23220153 Review.
-
Cav1.3 and Cav1.2 channels of adrenal chromaffin cells: emerging views on cAMP/cGMP-mediated phosphorylation and role in pacemaking.Biochim Biophys Acta. 2013 Jul;1828(7):1608-18. doi: 10.1016/j.bbamem.2012.11.013. Epub 2012 Nov 15. Biochim Biophys Acta. 2013. PMID: 23159773 Review.
Cited by
-
Integration of Rap1 and Calcium Signaling.Int J Mol Sci. 2020 Feb 27;21(5):1616. doi: 10.3390/ijms21051616. Int J Mol Sci. 2020. PMID: 32120817 Free PMC article. Review.
-
Insulin secretory actions of ethanolic extract of Acacia arabica bark in high fat-fed diet-induced obese Type 2 diabetic rats.Biosci Rep. 2023 May 31;43(5):BSR20230329. doi: 10.1042/BSR20230329. Biosci Rep. 2023. PMID: 37133312 Free PMC article.
-
The Effects of Dual GLP-1/GIP Receptor Agonism on Glucagon Secretion-A Review.Int J Mol Sci. 2019 Aug 22;20(17):4092. doi: 10.3390/ijms20174092. Int J Mol Sci. 2019. PMID: 31443356 Free PMC article. Review.
-
Tibetan Medicine for Diabetes Mellitus: Overview of Pharmacological Perspectives.Front Pharmacol. 2021 Oct 21;12:748500. doi: 10.3389/fphar.2021.748500. eCollection 2021. Front Pharmacol. 2021. PMID: 34744728 Free PMC article. Review.
-
D-Pinitol from Ceratonia siliqua Is an Orally Active Natural Inositol That Reduces Pancreas Insulin Secretion and Increases Circulating Ghrelin Levels in Wistar Rats.Nutrients. 2020 Jul 8;12(7):2030. doi: 10.3390/nu12072030. Nutrients. 2020. PMID: 32650579 Free PMC article.
References
-
- Arnette D, Gibson TB, Lawrence MC, January B, Khoo S, McGlynn K, Vanderbilt CA, Cobb MH. Regulation of ERK1 and ERK2 by glucose and peptide hormones in pancreatic beta cells. J Biol Chem. 2003;278(35):32517–32525. - PubMed
-
- Asfari M, Janjic D, Meda P, Li G, Halban PA, Wollheim CB. Establishment of 2-mercaptoethanol-dependent differentiated insulin-secreting cell lines. Endocrinology. 1992;130(1):167–178. - PubMed
-
- Bashan N, Dorfman K, Tarnovscki T, Harman-Boehm I, Liberty IF, Bluher M, Ovadia S, Maymon-Zilberstein T, Potashnik R, Stumvoll M, Avinoach E, Rudich A. Mitogen-activated protein kinases, inhibitory-kappaB kinase, and insulin signaling in human omental versus subcutaneous adipose tissue in obesity. Endocrinology. 2007;148(6):2955–2962. - PubMed
-
- Bouzakri K, Roques M, Gual P, Espinosa S, Guebre-Egziabher F, Riou JP, Laville M, Le Marchand-Brustel Y, Tanti JF, Vidal H. Reduced activation of phosphatidylinositol-3 kinase and increased serine 636 phosphorylation of insulin receptor substrate-1 in primary culture of skeletal muscle cells from patients with type 2 diabetes. Diabetes. 2003;52(6):1319–1325. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

