Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics
- PMID: 26432660
- PMCID: PMC4596920
- DOI: 10.1016/j.redox.2015.08.013
Nitric oxide-mediated sensitization of resistant tumor cells to apoptosis by chemo-immunotherapeutics
Abstract
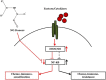
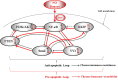
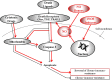
The generation of NO by the various NO synthases in normal and malignant tissues is manifested by various biological effects that are involved in the regulation of cell survival, differentiation and cell death. The role of NO in the cytotoxic immune response was first revealed by demonstrating the induction of iNOS in target cells by immune cytokines (e.g. IFN-γ, IL-1, TNF-α, etc.) and resulting in the sensitization of resistant tumor cells to death ligands-induced apoptosis. Endogenous/exogenous NO mediated its immune sensitizing effect by inhibiting NF-κΒ activity and downstream, inactivating the repressor transcription factor YY1, which inhibited both Fas and DR5 expressions. In addition, NO-mediated inhibition of NF-κΒ activity and inhibition downstream of its anti-apoptotic gene targets sensitized the tumor cells to apoptosis by chemotherapeutic drugs. We have identified in tumor cells a dysregulated pro-survival/anti-apoptotic loop consisting of NF-κB/Snail/YY1/RKIP/PTEN and its modification by NO was responsible, in large, for the reversal of chemo and immune resistance and sensitization to apoptotic mechanisms by cytotoxic agents. Moreover, tumor cells treated with exogenous NO donors resulted in the inhibition of NF-κΒ activity via S-nitrosylation of p50 and p65, inhibition of Snail (NF-κΒ target gene), inhibition of transcription repression by S-nitrosylation of YY1 and subsequent inhibition of epithelial-mesenchymal transition (EMT), induction of RKIP (inhibition of the transcription repressor Snail), and induction of PTEN (inhibition of the repressors Snail and YY1). Further, each gene product modified by NO in the loop was involved in chemo-immunosensitization. These above findings demonstrated that NO donors interference in the regulatory circuitry result in chemo-immunosensitization and inhibition of EMT. Overall, these observations suggest the potential anti-tumor therapeutic effect of NO donors in combination with subtoxic chemo-immuno drugs. This combination acts on multiple facets including reversal of chemo-immune resistance, and inhibition of both EMT and metastasis.
Keywords: Apoptosis; Chemotherapeutic drugs; Nitric oxide; Nitrosylation; Sensitization; Trail DR5.
Copyright © 2015 The Authors. Published by Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Dual role of NO donors in the reversal of tumor cell resistance and EMT: Downregulation of the NF-κB/Snail/YY1/RKIP circuitry.Nitric Oxide. 2011 Jan 1;24(1):1-7. doi: 10.1016/j.niox.2010.10.001. Epub 2010 Oct 8. Nitric Oxide. 2011. PMID: 20933602 Review.
-
Regulation Of Cell Death Apoptotic Pathways By Nitric Oxide In Cancer: Reversal Of Drug/Immune Resistance.Redox Biol. 2015 Aug;5:415. doi: 10.1016/j.redox.2015.09.016. Epub 2015 Dec 30. Redox Biol. 2015. PMID: 28162274
-
Novel therapeutic applications of nitric oxide donors in cancer: roles in chemo- and immunosensitization to apoptosis and inhibition of metastases.Nitric Oxide. 2008 Sep;19(2):152-7. doi: 10.1016/j.niox.2008.04.018. Epub 2008 Apr 24. Nitric Oxide. 2008. PMID: 18477483 Review.
-
The anti-CD20 mAb LFB-R603 interrupts the dysregulated NF-κB/Snail/RKIP/PTEN resistance loop in B-NHL cells: role in sensitization to TRAIL apoptosis.Int J Oncol. 2011 Jun;38(6):1683-94. doi: 10.3892/ijo.2011.984. Epub 2011 Mar 23. Int J Oncol. 2011. PMID: 21455568
-
RKIP-mediated chemo-immunosensitization of resistant cancer cells via disruption of the NF-κB/Snail/YY1/RKIP resistance-driver loop.Crit Rev Oncog. 2014;19(6):431-45. doi: 10.1615/critrevoncog.2014011929. Crit Rev Oncog. 2014. PMID: 25597353 Free PMC article. Review.
Cited by
-
S-Nitrosylation in Tumor Microenvironment.Int J Mol Sci. 2021 Apr 27;22(9):4600. doi: 10.3390/ijms22094600. Int J Mol Sci. 2021. PMID: 33925645 Free PMC article. Review.
-
Nitric oxide-releasing nanoparticles improve doxorubicin anticancer activity.Int J Nanomedicine. 2018 Nov 20;13:7771-7787. doi: 10.2147/IJN.S187089. eCollection 2018. Int J Nanomedicine. 2018. PMID: 30538458 Free PMC article.
-
Increasing intratumor C/EBP-β LIP and nitric oxide levels overcome resistance to doxorubicin in triple negative breast cancer.J Exp Clin Cancer Res. 2018 Nov 27;37(1):286. doi: 10.1186/s13046-018-0967-0. J Exp Clin Cancer Res. 2018. PMID: 30482226 Free PMC article.
-
Targeting Transcription Factor YY1 for Cancer Treatment: Current Strategies and Future Directions.Cancers (Basel). 2023 Jul 5;15(13):3506. doi: 10.3390/cancers15133506. Cancers (Basel). 2023. PMID: 37444616 Free PMC article. Review.
-
The biological implications of Yin Yang 1 in the hallmarks of cancer.Theranostics. 2020 Mar 4;10(9):4183-4200. doi: 10.7150/thno.43481. eCollection 2020. Theranostics. 2020. PMID: 32226547 Free PMC article. Review.
References
-
- Aggarwal B.B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 2003;3:745–756. - PubMed
-
- Aggarwal B.B. Nuclear factor-kappa-B: the enemy within. Cancer Cell. 2004;6:203–208. - PubMed
-
- Binder C., Schulz M., Hiddemann W., Oellerich M. Induction of inducible nitric oxide synthase is an essential part of tumor necrosis factor-alpha-induced apoptosis in MCF-7 and other epithelial tumor cells. Lab. Investig. 1999;79:1703–1712. - PubMed
-
- Bredt D.S. Endogenous nitric oxide synthesis: biological functions and pathophysiology. Free Radic. Res. 1999;31:577–596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

