Interplay between the virus and the ubiquitin-proteasome system: molecular mechanism of viral pathogenesis
- PMID: 26426962
- PMCID: PMC7102833
- DOI: 10.1016/j.coviro.2015.09.005
Interplay between the virus and the ubiquitin-proteasome system: molecular mechanism of viral pathogenesis
Abstract
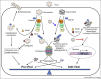
The ubiquitin-proteasome system (UPS) plays a central role in a wide range of fundamental cellular functions by ensuring protein quality control and through maintaining a critical level of important regulatory proteins. Viruses subvert or manipulate this cellular machinery to favor viral propagation and to evade host immune response. The UPS serves as a double-edged sword in viral pathogenesis: on the one hand, the UPS is utilized by many viruses to maintain proper function and level of viral proteins; while on the other hand, the UPS constitutes a host defense mechanism to eliminate viral components. To combat this host anti-viral machinery, viruses have evolved to employ the UPS to degrade or inactivate cellular proteins that limit viral growth. This review will highlight our current knowledge pertaining to the different roles for the UPS in viral pathogenesis.
Copyright © 2015 Elsevier B.V. All rights reserved.
Figures
Similar articles
-
Pleiotropic roles of the ubiquitin-proteasome system during viral propagation.Life Sci. 2018 Aug 15;207:350-354. doi: 10.1016/j.lfs.2018.06.014. Epub 2018 Jun 18. Life Sci. 2018. PMID: 29913185 Free PMC article. Review.
-
The interaction of hepatitis B virus with the ubiquitin proteasome system in viral replication and associated pathogenesis.Virol J. 2019 May 30;16(1):73. doi: 10.1186/s12985-019-1183-z. Virol J. 2019. PMID: 31146743 Free PMC article. Review.
-
The ubiquitin-proteasome system in positive-strand RNA virus infection.Rev Med Virol. 2013 Mar;23(2):85-96. doi: 10.1002/rmv.1725. Epub 2012 Jul 11. Rev Med Virol. 2013. PMID: 22782620 Free PMC article. Review.
-
Hijacking of the Ubiquitin/Proteasome Pathway by the HIV Auxiliary Proteins.Viruses. 2017 Oct 31;9(11):322. doi: 10.3390/v9110322. Viruses. 2017. PMID: 29088112 Free PMC article. Review.
-
Plant Virus Infection and the Ubiquitin Proteasome Machinery: Arms Race along the Endoplasmic Reticulum.Viruses. 2016 Nov 19;8(11):314. doi: 10.3390/v8110314. Viruses. 2016. PMID: 27869775 Free PMC article. Review.
Cited by
-
Virus-host protein co-expression networks reveal temporal organization and strategies of viral infection.iScience. 2023 Nov 16;26(12):108475. doi: 10.1016/j.isci.2023.108475. eCollection 2023 Dec 15. iScience. 2023. PMID: 38077135 Free PMC article.
-
Upregulated Proteasome Subunits in COVID-19 Patients: A Link with Hypoxemia, Lymphopenia and Inflammation.Biomolecules. 2022 Mar 13;12(3):442. doi: 10.3390/biom12030442. Biomolecules. 2022. PMID: 35327634 Free PMC article.
-
Dual-Role Ubiquitination Regulation Shuttling the Entire Life Cycle of the Flaviviridae.Front Microbiol. 2022 May 6;13:835344. doi: 10.3389/fmicb.2022.835344. eCollection 2022. Front Microbiol. 2022. PMID: 35602051 Free PMC article. Review.
-
K48-linked polyubiquitination of dengue virus NS1 protein inhibits its interaction with the viral partner NS4B.Virus Res. 2018 Feb 15;246:1-11. doi: 10.1016/j.virusres.2017.12.013. Epub 2017 Dec 30. Virus Res. 2018. PMID: 29294313 Free PMC article.
-
Structural dynamics of the E6AP/UBE3A-E6-p53 enzyme-substrate complex.Nat Commun. 2018 Oct 25;9(1):4441. doi: 10.1038/s41467-018-06953-0. Nat Commun. 2018. PMID: 30361475 Free PMC article.
References
-
- Glickman M.H., Ciechanover A. The ubiquitin–proteasome proteolytic pathway: destruction for the sake of construction. Physiol Rev. 2002;82:373–428. - PubMed
-
- Weissman A.M. Themes and variations on ubiquitylation. Nat Rev Mol Cell Biol. 2001;2:169–178. - PubMed
-
- Johnson E.S. Ubiquitin branches out. Nat Cell Biol. 2002;4:E295–E298. - PubMed
-
- Flotho A., Melchior F. Sumoylation: a regulatory protein modification in health and disease. Annu Rev Biochem. 2013;82:357–385. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

