The Effect of Transient Local Anti-inflammatory Treatment on the Survival of Pig Retinal Progenitor Cell Allotransplants
- PMID: 26425402
- PMCID: PMC4585327
- DOI: 10.1167/tvst.4.5.6
The Effect of Transient Local Anti-inflammatory Treatment on the Survival of Pig Retinal Progenitor Cell Allotransplants
Abstract
Purpose: The development of photoreceptor replacement therapy for retinal degenerative disorders requires the identification of the optimal cell source and immunosuppressive regimen in a large animal model. Allotransplants are not acutely rejected in swine subretinal space, although it is not known if survival can be improved with immunosuppression. Here we investigated the survival and integration of expanded pig retinal progenitor cells (pRPCs) in normal recipients with and without transient anti-inflammatory suppression.
Methods: pRPCs were derived from the neural retina of E60 GFP transgenic pigs, expanded for six passages, characterized, and transplanted into the subretinal space of 12 pigs. Six recipients received a single intravitreal injection of rapamycin and dexamethasone.
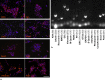
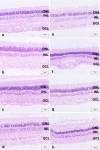
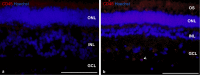
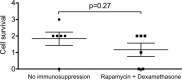
Results: pRPCs expressed the photoreceptor development genes Sox2, Pax6, Lhx2, Crx, Nrl, and Recoverin in vitro. Transplanted cells were identified in 9 out of 12 recipients 4 weeks after the injection. pRPCs integrated primarily into the photoreceptor inner segment layer and outer nuclear layer with single cells present in the inner nuclear layer. Donor cells remained recoverin-positive and acquired rhodopsin. We did not observe any signs of graft proliferation. The immunosuppression did not affect the survival or distribution of grafts. No macrophage infiltration or loss of retinal structure was observed in either group.
Conclusions: Local immunosuppression with rapamycin and dexamethasone does not improve the outcome of pRPC allotransplantation into the subretinal space.
Translational relevance: Survival and integration of pRPC together with the lack of graft proliferation suggests that allogeneic RPC transplantation without transient immunosuppression is a favorable approach for photoreceptor cell replacement.
Keywords: cell therapy; photoreceptors; rapamycin; retina; retinal progenitor cells.
Figures

Similar articles
-
Long-term survival and differentiation of retinal neurons derived from human embryonic stem cell lines in un-immunosuppressed mouse retina.Mol Vis. 2012;18:920-36. Epub 2012 Apr 12. Mol Vis. 2012. PMID: 22539871 Free PMC article.
-
Photoreceptor differentiation and integration of retinal progenitor cells transplanted into transgenic rats.Exp Eye Res. 2005 Apr;80(4):515-25. doi: 10.1016/j.exer.2004.11.001. Exp Eye Res. 2005. PMID: 15781279
-
Progenitor cells from the porcine neural retina express photoreceptor markers after transplantation to the subretinal space of allorecipients.Stem Cells. 2007 May;25(5):1222-30. doi: 10.1634/stemcells.2006-0541. Epub 2007 Jan 11. Stem Cells. 2007. PMID: 17218397
-
Iris pigment epithelial cell transplantation for degenerative retinal diseases.Prog Retin Eye Res. 2007 May;26(3):302-21. doi: 10.1016/j.preteyeres.2007.01.003. Epub 2007 Jan 17. Prog Retin Eye Res. 2007. PMID: 17324604 Review.
-
Transplantation of cultured progenitor cells to the mammalian retina.Expert Opin Biol Ther. 2006 May;6(5):443-51. doi: 10.1517/14712598.6.5.443. Expert Opin Biol Ther. 2006. PMID: 16610975 Review.
Cited by
-
Cellular regeneration strategies for macular degeneration: past, present and future.Eye (Lond). 2018 May;32(5):946-971. doi: 10.1038/s41433-018-0061-z. Epub 2018 Mar 5. Eye (Lond). 2018. PMID: 29503449 Free PMC article. Review.
-
Delivery Systems in Ocular Retinopathies: The Promising Future of Intravitreal Hydrogels as Sustained-Release Scaffolds.Pharmaceutics. 2023 May 12;15(5):1484. doi: 10.3390/pharmaceutics15051484. Pharmaceutics. 2023. PMID: 37242726 Free PMC article. Review.
-
Dexamethasone Provides Effective Immunosuppression for Improved Survival of Retinal Organoids after Epiretinal Transplantation.Stem Cells Int. 2019 Jul 25;2019:7148032. doi: 10.1155/2019/7148032. eCollection 2019. Stem Cells Int. 2019. PMID: 31428159 Free PMC article.
-
Production of a Locus- and Allele-Specific Monoclonal Antibody for the Characterization of SLA-1*0401 mRNA and Protein Expression Levels in MHC-Defined Microminipigs.PLoS One. 2016 Oct 19;11(10):e0164995. doi: 10.1371/journal.pone.0164995. eCollection 2016. PLoS One. 2016. PMID: 27760184 Free PMC article.
-
In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation.Cell Transplant. 2019 May;28(5):596-606. doi: 10.1177/0963689719825614. Epub 2019 Mar 27. Cell Transplant. 2019. PMID: 30917696 Free PMC article.
References
-
- MacLaren RE Pearson RA, MacNeil A,, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006; 444: 203–207. - PubMed
-
- Aftab U Jiang C, Tucker B,, et al. Growth kinetics and transplantation of human retinal progenitor cells. Exp Eye Res. 2009; 89: 301– 310. - PubMed
-
- Schwartz SD Hubschman JP, Heilwell G,, et al. Embryonic stem cell trials for macular degeneration: a preliminary report. Lancet. 2012; 379: 713– 720. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

