Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer's Disease
- PMID: 26424877
- PMCID: PMC4588605
- DOI: 10.1523/JNEUROSCI.1034-15.2015
Neuronal Store-Operated Calcium Entry and Mushroom Spine Loss in Amyloid Precursor Protein Knock-In Mouse Model of Alzheimer's Disease
Abstract
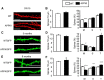
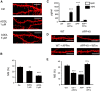
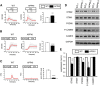
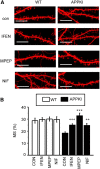
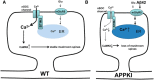
Alzheimer's disease (AD) is the most common reason for elderly dementia in the world. We proposed that memory loss in AD is related to destabilization of mushroom postsynaptic spines involved in long-term memory storage. We demonstrated previously that stromal interaction molecule 2 (STIM2)-regulated neuronal store-operated calcium entry (nSOC) in postsynaptic spines play a key role in stability of mushroom spines by maintaining activity of synaptic Ca(2+)/calmodulin kinase II (CaMKII). Furthermore, we demonstrated previously that the STIM2-nSOC-CaMKII pathway is downregulated in presenilin 1 M146V knock-in (PS1-M146V KI) mouse model of AD, leading to loss of hippocampal mushroom spines in this model. In the present study, we demonstrate that hippocampal mushroom postsynaptic spines are also lost in amyloid precursor protein knock-in (APPKI) mouse model of AD. We demonstrated that loss of mushroom spines occurs as a result of accumulation of extracellular β-amyloid 42 in APPKI culture media. Our results indicate that extracellular Aβ42 acts by overactivating mGluR5 receptor in APPKI neurons, leading to elevated Ca(2+) levels in endoplasmic reticulum, compensatory downregulation of STIM2 expression, impaired synaptic nSOC, and reduced CaMKII activity. Pharmacological inhibition of mGluR5 or overexpression of STIM2 rescued synaptic nSOC and prevented mushroom spine loss in APPKI hippocampal neurons. Our results indicate that downregulation of synaptic STIM2-nSOC-CaMKII pathway causes loss of mushroom synaptic spines in both presenilin and APPKI mouse models of AD. We propose that modulators/activators of this pathway may have a potential therapeutic value for treatment of memory loss in AD. Significance statement: A direct connection between amyloid-induced synaptic mushroom spine loss and neuronal store-operated calcium entry pathway is shown. These results provide strong support for the calcium hypothesis of neurodegeneration and further validate the synaptic store-operated calcium entry pathway as a potential therapeutic target for Alzheimer's disease.
Keywords: calcium; imaging; synaptic; transgenic.
Copyright © 2015 the authors 0270-6474/15/3513275-12$15.00/0.
Figures
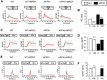
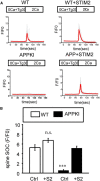
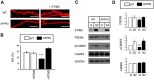
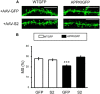
Similar articles
-
Store-Operated Calcium Channel Complex in Postsynaptic Spines: A New Therapeutic Target for Alzheimer's Disease Treatment.J Neurosci. 2016 Nov 23;36(47):11837-11850. doi: 10.1523/JNEUROSCI.1188-16.2016. J Neurosci. 2016. PMID: 27881772 Free PMC article.
-
Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice.Neuron. 2014 Apr 2;82(1):79-93. doi: 10.1016/j.neuron.2014.02.019. Neuron. 2014. PMID: 24698269 Free PMC article.
-
Stim2-Eb3 Association and Morphology of Dendritic Spines in Hippocampal Neurons.Sci Rep. 2017 Dec 15;7(1):17625. doi: 10.1038/s41598-017-17762-8. Sci Rep. 2017. PMID: 29247211 Free PMC article.
-
Dysregulation of neuronal calcium homeostasis in Alzheimer's disease - A therapeutic opportunity?Biochem Biophys Res Commun. 2017 Feb 19;483(4):998-1004. doi: 10.1016/j.bbrc.2016.09.053. Epub 2016 Sep 15. Biochem Biophys Res Commun. 2017. PMID: 27641664 Free PMC article. Review.
-
Drebrin in Alzheimer's Disease.Adv Exp Med Biol. 2017;1006:203-223. doi: 10.1007/978-4-431-56550-5_12. Adv Exp Med Biol. 2017. PMID: 28865022 Review.
Cited by
-
Presenilin 1 Regulates [Ca2+]i and Mitochondria/ER Interaction in Cultured Rat Hippocampal Neurons.Oxid Med Cell Longev. 2019 Jul 28;2019:7284967. doi: 10.1155/2019/7284967. eCollection 2019. Oxid Med Cell Longev. 2019. PMID: 31467635 Free PMC article.
-
STIM and Orai Mediated Regulation of Calcium Signaling in Age-Related Diseases.Front Aging. 2022 Apr 19;3:876785. doi: 10.3389/fragi.2022.876785. eCollection 2022. Front Aging. 2022. PMID: 35821821 Free PMC article. Review.
-
STIM Protein-NMDA2 Receptor Interaction Decreases NMDA-Dependent Calcium Levels in Cortical Neurons.Cells. 2020 Jan 9;9(1):160. doi: 10.3390/cells9010160. Cells. 2020. PMID: 31936514 Free PMC article.
-
Reduced uptake of [11C]-ABP688, a PET tracer for metabolic glutamate receptor 5 in hippocampus and amygdala in Alzheimer's dementia.Brain Behav. 2020 Jun;10(6):e01632. doi: 10.1002/brb3.1632. Epub 2020 Apr 18. Brain Behav. 2020. PMID: 32304284 Free PMC article.
-
Rodent Models of Alzheimer's Disease: Past Misconceptions and Future Prospects.Int J Mol Sci. 2024 Jun 5;25(11):6222. doi: 10.3390/ijms25116222. Int J Mol Sci. 2024. PMID: 38892408 Free PMC article. Review.
References
-
- De Felice FG, Velasco PT, Lambert MP, Viola K, Fernandez SJ, Ferreira ST, Klein WL. Abeta oligomers induce neuronal oxidative stress through an N-methyl-d-aspartate receptor-dependent mechanism that is blocked by the Alzheimer drug memantine. J Biol Chem. 2007;282:11590–11601. doi: 10.1074/jbc.M607483200. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
