Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity
- PMID: 26418893
- PMCID: PMC4755868
- DOI: 10.1002/stem.2213
Definitive Hematopoiesis in the Yolk Sac Emerges from Wnt-Responsive Hemogenic Endothelium Independently of Circulation and Arterial Identity
Abstract
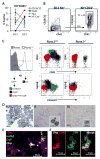
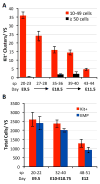
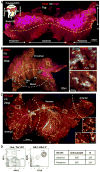
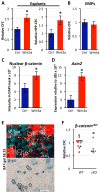
Adult-repopulating hematopoietic stem cells (HSCs) emerge in low numbers in the midgestation mouse embryo from a subset of arterial endothelium, through an endothelial-to-hematopoietic transition. HSC-producing arterial hemogenic endothelium relies on the establishment of embryonic blood flow and arterial identity, and requires β-catenin signaling. Specified prior to and during the formation of these initial HSCs are thousands of yolk sac-derived erythro-myeloid progenitors (EMPs). EMPs ensure embryonic survival prior to the establishment of a permanent hematopoietic system, and provide subsets of long-lived tissue macrophages. While an endothelial origin for these HSC-independent definitive progenitors is also accepted, the spatial location and temporal output of yolk sac hemogenic endothelium over developmental time remain undefined. We performed a spatiotemporal analysis of EMP emergence, and document the morphological steps of the endothelial-to-hematopoietic transition. Emergence of rounded EMPs from polygonal clusters of Kit(+) cells initiates prior to the establishment of arborized arterial and venous vasculature in the yolk sac. Interestingly, Kit(+) polygonal clusters are detected in both arterial and venous vessels after remodeling. To determine whether there are similar mechanisms regulating the specification of EMPs with other angiogenic signals regulating adult-repopulating HSCs, we investigated the role of embryonic blood flow and Wnt/β-catenin signaling during EMP emergence. In embryos lacking a functional circulation, rounded Kit(+) EMPs still fully emerge from unremodeled yolk sac vasculature. In contrast, canonical Wnt signaling appears to be a common mechanism regulating hematopoietic emergence from hemogenic endothelium. These data illustrate the heterogeneity in hematopoietic output and spatiotemporal regulation of primary embryonic hemogenic endothelium.
Keywords: Embryo; Endothelial cell; Hemangioblast; Hematopoiesis; Hematopoietic progenitors; Hematopoietic stem cells; Vascular development.
© 2015 AlphaMed Press.
Conflict of interest statement
The authors have no conflicts of interest.
Figures


Similar articles
-
Lack of Phenotypical and Morphological Evidences of Endothelial to Hematopoietic Transition in the Murine Embryonic Head during Hematopoietic Stem Cell Emergence.PLoS One. 2016 May 26;11(5):e0156427. doi: 10.1371/journal.pone.0156427. eCollection 2016. PLoS One. 2016. PMID: 27227884 Free PMC article.
-
Hlf marks the developmental pathway for hematopoietic stem cells but not for erythro-myeloid progenitors.J Exp Med. 2019 Jul 1;216(7):1599-1614. doi: 10.1084/jem.20181399. Epub 2019 May 10. J Exp Med. 2019. PMID: 31076455 Free PMC article.
-
Erythro-myeloid progenitors can differentiate from endothelial cells and modulate embryonic vascular remodeling.Sci Rep. 2017 Mar 8;7:43817. doi: 10.1038/srep43817. Sci Rep. 2017. PMID: 28272478 Free PMC article.
-
From hemangioblast to hematopoietic stem cell: an endothelial connection?Exp Hematol. 2005 Sep;33(9):1029-40. doi: 10.1016/j.exphem.2005.06.005. Exp Hematol. 2005. PMID: 16140151 Review.
-
The embryonic origins of hematopoietic stem cells: a tale of hemangioblast and hemogenic endothelium.APMIS. 2005 Nov-Dec;113(11-12):790-803. doi: 10.1111/j.1600-0463.2005.apm_317.x. APMIS. 2005. PMID: 16480450 Review.
Cited by
-
Generating human hematopoietic stem cells in vitro -exploring endothelial to hematopoietic transition as a portal for stemness acquisition.FEBS Lett. 2016 Nov;590(22):4126-4143. doi: 10.1002/1873-3468.12283. Epub 2016 Jul 22. FEBS Lett. 2016. PMID: 27391301 Free PMC article. Review.
-
Extravascular endothelial and hematopoietic islands form through multiple pathways in midgestation mouse embryos.Dev Biol. 2016 Jul 1;415(1):111-121. doi: 10.1016/j.ydbio.2016.04.004. Epub 2016 Apr 20. Dev Biol. 2016. PMID: 27105579 Free PMC article.
-
Toll-like receptor 2 expression on c-kit+ cells tracks the emergence of embryonic definitive hematopoietic progenitors.Nat Commun. 2019 Nov 15;10(1):5176. doi: 10.1038/s41467-019-13150-0. Nat Commun. 2019. PMID: 31729371 Free PMC article.
-
Arterial identity of hemogenic endothelium: a key to unlock definitive hematopoietic commitment in human pluripotent stem cell cultures.Exp Hematol. 2019 Mar;71:3-12. doi: 10.1016/j.exphem.2018.11.007. Epub 2018 Nov 28. Exp Hematol. 2019. PMID: 30500414 Free PMC article. Review.
-
Fate-Mapping Macrophages: From Ontogeny to Functions.Methods Mol Biol. 2024;2713:11-43. doi: 10.1007/978-1-0716-3437-0_2. Methods Mol Biol. 2024. PMID: 37639113 Review.
References
-
- Muller AM, Medvinsky A, Strouboulis J, et al. Development of hematopoietic stem cell activity in the mouse embryo. Immunity. 1994;1:291–301. - PubMed
-
- Okuda T, Deursen J, van Hiebert SW, et al. AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell. 1996;84:321–330. - PubMed
-
- North T, Gu TL, Stacy T, et al. Cbfa2 is required for the formation of intra-aortic hematopoietic clusters. Development. 1999;126(11):2563–2575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

