Selective Regulation of Oocyte Meiotic Events Enhances Progress in Fertility Preservation Methods
- PMID: 26417205
- PMCID: PMC4577271
- DOI: 10.4137/BCI.S28596
Selective Regulation of Oocyte Meiotic Events Enhances Progress in Fertility Preservation Methods
Abstract
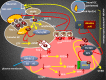
Following early embryonic germ cell migration, oocytes are surrounded by somatic cells and remain arrested at diplotene stage until luteinizing hormone (LH) surge. Strict regulation of both meiotic arrest and meiotic resumption during dormant stage are critical for future fertility. Inter-cellular signaling system between the somatic compartment and oocyte regulates these meiotic events and determines the follicle quality. As well as the collected number of eggs, their qualities are also important for in vitro fertilization (IVF) outcome. In spontaneous and IVF cycles, germinal vesicle (GV)-stage oocytes, premature GV breakdown, and persistence of first meiotic arrest limit the reproductive performance. Likewise, both women with premature ovarian aging and young cancer women are undergoing chemoradiotherapy under the risk of follicle loss because of unregulated meiotic events. Understanding of oocyte meiotic events is therefore critical for the prevention of functional ovarian reserve. High levels of cyclic guanosine monophophate (cGMP), cyclic adenosine monophophate (cAMP) and low phosphodiesterase (PDE) 3A enzyme activity inside the oocyte are responsible for maintaining of meiotic arrest before the LH surge. cGMP is produced in the somatic compartment, and natriuretic peptide precursor C (Nppc) and natriuretic peptide receptor 2 (Npr2) regulate its production. cGMP diffuses into the oocyte and reduces the PDE3A activity, which inhibits the conversion of cAMP to the 5'AMP, and cAMP levels are enhanced. In addition, oocyte itself has the ability to produce cAMP. Taken together, accumulation of cAMP inside the oocyte induces protein kinase activity, which leads to the inhibition of maturation-promoting factor and meiotic arrest also continues. By stimulating the expression of epidermal growth factor, LH inhibits the Nppc/Npr2 system, blocks cGMP synthesis, and initiates meiotic resumption. Oocytes lacking the functional of this pathway may lead to persistence of the GV oocyte, which reduces the number of good quality eggs. Selective regulation of somatic cell signals and oocyte meiotic events enhance progress in fertility preservation methods, which may give us the opportunity to prevent follicle loss in prematurely aging women and young women with cancer are undergoing chemoradiotherapy.
Keywords: Nppc/Npr2; PDEs; cAMP; cGMP; fertility preservation; oocyte meiosis.
Figures


Similar articles
-
Nppc/Npr2/cGMP signaling cascade maintains oocyte developmental capacity.Cell Mol Biol (Noisy-le-grand). 2019 Apr 30;65(4):83-89. Cell Mol Biol (Noisy-le-grand). 2019. PMID: 31078160 Review.
-
Hormonal control of mammalian oocyte meiosis at diplotene stage.Cell Mol Life Sci. 2012 Apr;69(8):1279-88. doi: 10.1007/s00018-011-0867-3. Epub 2011 Nov 2. Cell Mol Life Sci. 2012. PMID: 22045555 Free PMC article. Review.
-
Epidermal growth factor receptor signaling-dependent calcium elevation in cumulus cells is required for NPR2 inhibition and meiotic resumption in mouse oocytes.Endocrinology. 2013 Sep;154(9):3401-9. doi: 10.1210/en.2013-1133. Epub 2013 Jun 20. Endocrinology. 2013. PMID: 23787120
-
Towards a new understanding on the regulation of mammalian oocyte meiosis resumption.Cell Cycle. 2009 Sep 1;8(17):2741-7. doi: 10.4161/cc.8.17.9471. Epub 2009 Sep 8. Cell Cycle. 2009. PMID: 19717979 Review.
-
Signaling mechanisms and their regulation during in vivo or in vitro maturation of mammalian oocytes.Reprod Biol Endocrinol. 2022 Feb 24;20(1):37. doi: 10.1186/s12958-022-00906-5. Reprod Biol Endocrinol. 2022. PMID: 35209923 Free PMC article. Review.
Cited by
-
N-acetyl-L-cysteine (NAC) delays post-ovulatory oocyte aging in mouse.Aging (Albany NY). 2019 Apr 12;11(7):2020-2030. doi: 10.18632/aging.101898. Aging (Albany NY). 2019. PMID: 30978175 Free PMC article.
-
Whole genome sequencing of simmental cattle for SNP and CNV discovery.BMC Genomics. 2023 Apr 5;24(1):179. doi: 10.1186/s12864-023-09248-x. BMC Genomics. 2023. PMID: 37020271 Free PMC article.
-
Transforming growth factor-β is involved in maintaining oocyte meiotic arrest by promoting natriuretic peptide type C expression in mouse granulosa cells.Cell Death Dis. 2019 Jul 22;10(8):558. doi: 10.1038/s41419-019-1797-5. Cell Death Dis. 2019. PMID: 31332164 Free PMC article.
-
Selection signatures of litter size in Dazu black goats based on a whole genome sequencing mixed pools strategy.Mol Biol Rep. 2019 Oct;46(5):5517-5523. doi: 10.1007/s11033-019-04904-6. Epub 2019 Jun 7. Mol Biol Rep. 2019. PMID: 31175513
-
Unraveling the Puzzle: Oocyte Maturation Abnormalities (OMAS).Diagnostics (Basel). 2022 Oct 15;12(10):2501. doi: 10.3390/diagnostics12102501. Diagnostics (Basel). 2022. PMID: 36292190 Free PMC article. Review.
References
-
- Hunt PA, Hassold TJ. Human female meiosis: what makes a good egg go bad? Trends Genet. 2008;24(2):86–93. - PubMed
-
- Kishimoto T. Cell-cycle control during meiotic maturation. Curr Opin Cell Biol. 2003;15(6):654–663. - PubMed
-
- Whitaker M. Control of meiotic arrest. Rev Reprod. 1996;1(2):127–135. - PubMed
-
- Nishiyama T, Tachibana K, Kishimoto T. Cytostatic Arrest: Post- Ovulation Arrest until Fertilization in Metazoan Oocytes. In: Verlhac M-H, Villeneuve A, editors. Oogenesis: The Universal Process. John Wiley & Sons, Ltd; Chichester, UK: 2010. pp. 357–387.
-
- Adhikari D, Zheng W, Shen Y, et al. Cdk1, but not Cdk2, is the sole Cdk that is essential and sufficient to drive resumption of meiosis in mouse oocytes. Hum Mol Genet. 2012;21(11):2476–2484. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

