TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets
- PMID: 26417084
- PMCID: PMC4611630
- DOI: 10.1073/pnas.1516594112
TMEM16F is required for phosphatidylserine exposure and microparticle release in activated mouse platelets
Abstract
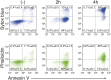
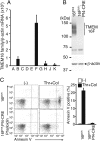
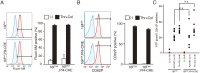
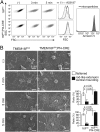
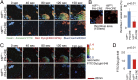
Phosphatidylserine (PtdSer) exposure on the surface of activated platelets requires the action of a phospholipid scramblase(s), and serves as a scaffold for the assembly of the tenase and prothrombinase complexes involved in blood coagulation. Here, we found that the activation of mouse platelets with thrombin/collagen or Ca(2+) ionophore at 20 °C induces PtdSer exposure without compromising plasma membrane integrity. Among five transmembrane protein 16 (TMEM16) members that support Ca(2+)-dependent phospholipid scrambling, TMEM16F was the only one that showed high expression in mouse platelets. Platelets from platelet-specific TMEM16F-deficient mice exhibited defects in activation-induced PtdSer exposure and microparticle shedding, although α-granule and dense granule release remained intact. The rate of tissue factor-induced thrombin generation by TMEM16F-deficient platelets was severely reduced, whereas thrombin-induced clot retraction was unaffected. The imaging of laser-induced thrombus formation in whole animals showed that PtdSer exposure on aggregated platelets was TMEM16F-dependent in vivo. The phenotypes of the platelet-specific TMEM16F-null mice resemble those of patients with Scott syndrome, a mild bleeding disorder, indicating that these mice may provide a useful model for human Scott syndrome.
Keywords: calcium; microvesicles; phosphatidylserine; platelets; scramblase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


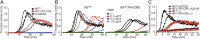

Similar articles
-
Both TMEM16F-dependent and TMEM16F-independent pathways contribute to phosphatidylserine exposure in platelet apoptosis and platelet activation.Blood. 2013 Mar 7;121(10):1850-7. doi: 10.1182/blood-2012-09-454314. Epub 2013 Jan 9. Blood. 2013. PMID: 23303820
-
A TMEM16F point mutation causes an absence of canine platelet TMEM16F and ineffective activation and death-induced phospholipid scrambling.J Thromb Haemost. 2015 Dec;13(12):2240-52. doi: 10.1111/jth.13157. Epub 2015 Nov 20. J Thromb Haemost. 2015. PMID: 26414452
-
Roles and regulation of phospholipid scramblases.FEBS Lett. 2015 Jan 2;589(1):3-14. doi: 10.1016/j.febslet.2014.11.036. Epub 2014 Dec 3. FEBS Lett. 2015. PMID: 25479087 Review.
-
Survival protein anoctamin-6 controls multiple platelet responses including phospholipid scrambling, swelling, and protein cleavage.FASEB J. 2016 Feb;30(2):727-37. doi: 10.1096/fj.15-280446. Epub 2015 Oct 19. FASEB J. 2016. PMID: 26481309
-
Platelet membrane phospholipid asymmetry: from the characterization of a scramblase activity to the identification of an essential protein mutated in Scott syndrome.J Thromb Haemost. 2011 Oct;9(10):1883-91. doi: 10.1111/j.1538-7836.2011.04478.x. J Thromb Haemost. 2011. PMID: 21958383 Review.
Cited by
-
Identification of a conserved drug binding pocket in TMEM16 proteins.Res Sq [Preprint]. 2022 Feb 10:rs.3.rs-1296933. doi: 10.21203/rs.3.rs-1296933/v1. Res Sq. 2022. Update in: Nat Commun. 2023 Aug 12;14(1):4874. doi: 10.1038/s41467-023-40410-x PMID: 35169791 Free PMC article. Updated. Preprint.
-
Gating and Regulatory Mechanisms of TMEM16 Ion Channels and Scramblases.Front Physiol. 2021 Nov 19;12:787773. doi: 10.3389/fphys.2021.787773. eCollection 2021. Front Physiol. 2021. PMID: 34867487 Free PMC article. Review.
-
Extracellular vesicles arising from apoptosis: forms, functions, and applications.J Pathol. 2023 Aug;260(5):592-608. doi: 10.1002/path.6138. Epub 2023 Jun 9. J Pathol. 2023. PMID: 37294158 Free PMC article. Review.
-
Molecular underpinning of intracellular pH regulation on TMEM16F.J Gen Physiol. 2021 Feb 1;153(2):e202012704. doi: 10.1085/jgp.202012704. J Gen Physiol. 2021. PMID: 33346788 Free PMC article.
-
Synergistic effects of EMPs and PMPs on pulmonary vascular leakage and lung injury after ischemia/reperfusion.Cell Commun Signal. 2020 Nov 23;18(1):184. doi: 10.1186/s12964-020-00672-0. Cell Commun Signal. 2020. PMID: 33225929 Free PMC article.
References
-
- Leventis PA, Grinstein S. The distribution and function of phosphatidylserine in cellular membranes. Annu Rev Biophys. 2010;39:407–427. - PubMed
-
- Balasubramanian K, Schroit AJ. Aminophospholipid asymmetry: A matter of life and death. Annu Rev Physiol. 2003;65:701–734. - PubMed
-
- Segawa K, et al. Caspase-mediated cleavage of phospholipid flippase for apoptotic phosphatidylserine exposure. Science. 2014;344(6188):1164–1168. - PubMed
-
- Suzuki J, Denning DP, Imanishi E, Horvitz HR, Nagata S. Xk-related protein 8 and CED-8 promote phosphatidylserine exposure in apoptotic cells. Science. 2013;341(6144):403–406. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

