A systematic review of training programmes for recruiters to randomised controlled trials
- PMID: 26416143
- PMCID: PMC4587840
- DOI: 10.1186/s13063-015-0908-6
A systematic review of training programmes for recruiters to randomised controlled trials
Abstract
Background: Recruitment to randomised controlled trials (RCTs) is often difficult. Clinician related factors have been implicated as important reasons for low rates of recruitment. Clinicians (doctors and other health professionals) can experience discomfort with some underlying principles of RCTs and experience difficulties in conveying them positively to potential trial participants. Recruiter training has been suggested to address identified problems but a synthesis of this research is lacking. The aim of our study was to systematically review the available evidence on training interventions for recruiters to randomised trials.
Methods: Studies that evaluated training programmes for trial recruiters were included. Those that provided only general communication training not linked to RCT recruitment were excluded. Data extraction and quality assessment were completed by two reviewers independently, with a third author where necessary.
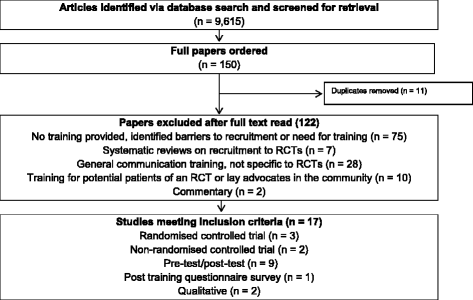
Results: Seventeen studies of 9615 potentially eligible titles and abstracts were included in the review: three randomised controlled studies, two non-randomised controlled studies, nine uncontrolled pre-test/post-test studies, two qualitative studies, and a post-training questionnaire survey. Most studies were of moderate or weak quality. Training programmes were mostly set within cancer trials, and usually consisted of workshops with a mix of health professionals over one or two consecutive days covering generic and trial specific issues. Recruiter training programmes were well received and some increased recruiters' self-confidence in communicating key RCT concepts to patients. There was, however, little evidence that this training increased actual recruitment rates or patient understanding, satisfaction, or levels of informed consent.
Conclusions: There is a need to develop recruiter training programmes that can lead to improved recruitment and informed consent in randomised trials.
Similar articles
-
Clear obstacles and hidden challenges: understanding recruiter perspectives in six pragmatic randomised controlled trials.Trials. 2014 Jan 6;15:5. doi: 10.1186/1745-6215-15-5. Trials. 2014. PMID: 24393291 Free PMC article.
-
Informed consent in randomised controlled trials: development and preliminary evaluation of a measure of Participatory and Informed Consent (PIC).Trials. 2017 Jul 17;18(1):327. doi: 10.1186/s13063-017-2048-7. Trials. 2017. PMID: 28716064 Free PMC article.
-
Evaluation of interventions for informed consent for randomised controlled trials (ELICIT): protocol for a systematic review of the literature and identification of a core outcome set using a Delphi survey.Trials. 2015 Oct 27;16:484. doi: 10.1186/s13063-015-1011-8. Trials. 2015. PMID: 26507504 Free PMC article.
-
Understanding and Improving Recruitment to Randomised Controlled Trials: Qualitative Research Approaches.Eur Urol. 2017 Nov;72(5):789-798. doi: 10.1016/j.eururo.2017.04.036. Epub 2017 Jun 1. Eur Urol. 2017. PMID: 28578829 Review.
-
Identifying the participant characteristics that predict recruitment and retention of participants to randomised controlled trials involving children: a systematic review.Trials. 2016 Jun 22;17(1):294. doi: 10.1186/s13063-016-1415-0. Trials. 2016. PMID: 27334018 Free PMC article. Review.
Cited by
-
Trials need participants but not their feedback? A scoping review of published papers on the measurement of participant experience of taking part in clinical trials.Trials. 2019 Jun 24;20(1):381. doi: 10.1186/s13063-019-3444-y. Trials. 2019. PMID: 31234945 Free PMC article. Review.
-
Exploring factors influencing recruitment results of nurses recruiting diabetes patients for a randomized controlled trial.Clin Trials. 2020 Aug;17(4):448-458. doi: 10.1177/1740774520914609. Epub 2020 May 5. Clin Trials. 2020. PMID: 32367737 Free PMC article.
-
Identifying research priorities for effective retention strategies in clinical trials.Trials. 2017 Aug 31;18(1):406. doi: 10.1186/s13063-017-2132-z. Trials. 2017. PMID: 28859674 Free PMC article.
-
Training health professionals to recruit into challenging randomized controlled trials improved confidence: the development of the QuinteT randomized controlled trial recruitment training intervention.J Clin Epidemiol. 2018 Mar;95:34-44. doi: 10.1016/j.jclinepi.2017.11.015. Epub 2017 Nov 27. J Clin Epidemiol. 2018. PMID: 29191445 Free PMC article.
-
Clinician-researchers and custodians of scarce resources: a qualitative study of health professionals' views on barriers to the involvement of teenagers and young adults in cancer trials.Trials. 2020 Jan 10;21(1):67. doi: 10.1186/s13063-019-3942-y. Trials. 2020. PMID: 31924260 Free PMC article.
References
-
- Donovan JL, de Salis I, Toerien M, Paramasivan S, Hamdy FC, Blazeby J. The intellectual challenges and emotional consequences of equipoise contributed to the fragility of recruitment in six randomized controlled trials. J Clin Epidemiol. 2014;67:912–20. doi: 10.1016/j.jclinepi.2014.03.010. - DOI - PMC - PubMed
-
- International Conference on Harmonisation . Guideline for good clinical practice E6. 1996.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources