Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy
- PMID: 26408543
- PMCID: PMC4666980
- DOI: 10.1152/ajpheart.00532.2015
Adaptive increases in expression and vasodilator activity of estrogen receptor subtypes in a blood vessel-specific pattern during pregnancy
Abstract
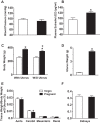
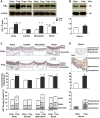
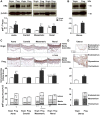
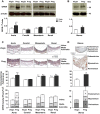
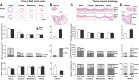
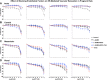
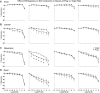
Normal pregnancy is associated with adaptive hemodynamic, hormonal, and vascular changes, and estrogen (E2) may promote vasodilation during pregnancy; however, the specific E2 receptor (ER) subtype, post-ER signaling mechanism, and vascular bed involved are unclear. We tested whether pregnancy-associated vascular adaptations involve changes in the expression/distribution/activity of distinct ER subtypes in a blood vessel-specific manner. Blood pressure (BP) and plasma E2 were measured in virgin and pregnant (day 19) rats, and the thoracic aorta, carotid artery, mesenteric artery, and renal artery were isolated for measurements of ERα, ERβ, and G protein-coupled receptor 30 [G protein-coupled ER (GPER)] expression and tissue distribution in parallel with relaxation responses to E2 (all ERs) and the specific ER agonist 4,4',4″-(4-propyl-[1H]-pyrazole-1,3,5-triyl)-tris-phenol (PPT; ERα), diarylpropionitrile (DPN; ERβ), and G1 (GPER). BP was slightly lower and plasma E2 was higher in pregnant versus virgin rats. Western blots revealed increased ERα and ERβ in the aorta and mesenteric artery and GPER in the aorta of pregnant versus virgin rats. Immunohistochemistry revealed that the increases in ERs were mainly in the intima and media. In phenylephrine-precontracted vessels, E2 and PPT caused relaxation that was greater in the aorta and mesenteric artery but similar in the carotid and renal artery of pregnant versus virgin rats. DPN- and G1-induced relaxation was greater in the mesenteric and renal artery than in the aorta and carotid artery, and aortic relaxation to G1 was greater in pregnant versus virgin rats. The nitric oxide synthase inhibitor N(ω)-nitro-l-arginine methyl ester with or without the cyclooxygenase inhibitor indomethacin with or without the EDHF blocker tetraethylammonium or endothelium removal reduced E2, PPT, and G1-induced relaxation in the aorta of pregnant rats, suggesting an endothelium-dependent mechanism, but did not affect E2-, PPT-, DPN-, or G1-induced relaxation in other vessels, suggesting endothelium-independent mechanisms. E2, PPT, DPN, and G1 caused relaxation of Ca(2+) entry-dependent KCl contraction, and the effect of PPT was greater in the mesenteric artery of pregnant versus virgin rats. Thus, during pregnancy, an increase in ERα expression in endothelial and vascular smooth muscle layers of the aorta and mesenteric artery is associated with increased ERα-mediated relaxation via endothelium-derived vasodilators and inhibition of Ca(2+) entry into vascular smooth muscle, supporting a role of aortic and mesenteric arterial ERα in pregnancy-associated vasodilation. GPER may contribute to aortic relaxation while enhanced ERβ expression could mediate other genomic vascular effects during pregnancy.
Keywords: calcium; endothelium; estrogen; nitric oxide; vascular smooth muscle.
Copyright © 2015 the American Physiological Society.
Figures

Similar articles
-
Estrogen receptor subtypes mediate distinct microvascular dilation and reduction in [Ca2+]I in mesenteric microvessels of female rat.J Pharmacol Exp Ther. 2015 Feb;352(2):291-304. doi: 10.1124/jpet.114.219865. Epub 2014 Dec 3. J Pharmacol Exp Ther. 2015. PMID: 25472954 Free PMC article.
-
Subtype-specific estrogen receptor-mediated vasodilator activity in the cephalic, thoracic, and abdominal vasculature of female rat.J Cardiovasc Pharmacol. 2013 Jul;62(1):26-40. doi: 10.1097/FJC.0b013e31828bc88a. J Cardiovasc Pharmacol. 2013. PMID: 23429596 Free PMC article.
-
Estrogen receptor-mediated enhancement of venous relaxation in female rat: implications in sex-related differences in varicose veins.J Vasc Surg. 2010 Apr;51(4):972-81. doi: 10.1016/j.jvs.2009.11.074. J Vasc Surg. 2010. PMID: 20347696 Free PMC article.
-
Estrogen Receptor Function: Impact on the Human Endometrium.Front Endocrinol (Lausanne). 2022 Feb 28;13:827724. doi: 10.3389/fendo.2022.827724. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35295981 Free PMC article. Review.
-
Review article: steroid hormones and uterine vascular adaptation to pregnancy.Reprod Sci. 2008 Apr;15(4):336-48. doi: 10.1177/1933719108317975. Reprod Sci. 2008. PMID: 18497342 Free PMC article. Review.
Cited by
-
Estrogen Actions in Placental Vascular Morphogenesis and Spiral Artery Remodeling: A Comparative View between Humans and Mice.Cells. 2023 Feb 14;12(4):620. doi: 10.3390/cells12040620. Cells. 2023. PMID: 36831287 Free PMC article. Review.
-
Immunohistochemical expression of estrogen receptor alpha in the maxillary sinus, pulp, and periodontal ligament of adjacent teeth in late pregnancy in rats.Odontology. 2023 Jul;111(3):608-617. doi: 10.1007/s10266-022-00770-0. Epub 2022 Nov 25. Odontology. 2023. PMID: 36434465 Free PMC article.
-
O-GlcNAc impairs endothelial function in uterine arteries from virgin but not pregnant rats: The role of GSK3β.Eur J Pharmacol. 2020 Aug 5;880:173133. doi: 10.1016/j.ejphar.2020.173133. Epub 2020 Apr 25. Eur J Pharmacol. 2020. PMID: 32343970 Free PMC article.
-
Restoring placental growth factor-soluble fms-like tyrosine kinase-1 balance reverses vascular hyper-reactivity and hypertension in pregnancy.Am J Physiol Regul Integr Comp Physiol. 2016 Sep 1;311(3):R505-21. doi: 10.1152/ajpregu.00137.2016. Epub 2016 Jun 8. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27280428 Free PMC article.
-
Decreased uterine vascularization and uterine arterial expansive remodeling with reduced matrix metalloproteinase-2 and -9 in hypertensive pregnancy.Am J Physiol Heart Circ Physiol. 2020 Jan 1;318(1):H165-H180. doi: 10.1152/ajpheart.00602.2019. Epub 2019 Dec 13. Am J Physiol Heart Circ Physiol. 2020. PMID: 31834839 Free PMC article.
References
-
- Aavik E, du Toit D, Myburgh E, Frosen J, Hayry P. Estrogen receptor beta dominates in baboon carotid after endothelial denudation injury. Mol Cell Endocrinol 182: 91–98, 2001. - PubMed
-
- Alda JO, Valero MS, Pereboom D, Gros P, Garay RP. Endothelium-independent vasorelaxation by the selective alpha estrogen receptor agonist propyl pyrazole triol in rat aortic smooth muscle. J Pharm Pharmacol 61: 641–646, 2009. - PubMed
-
- Barron LA, Giardina JB, Granger JP, Khalil RA. High-salt diet enhances vascular reactivity in pregnant rats with normal and reduced uterine perfusion pressure. Hypertension 38: 730–735, 2001. - PubMed
-
- Bolego C, Cignarella A, Sanvito P, Pelosi V, Pellegatta F, Puglisi L, Pinna C. The acute estrogenic dilation of rat aorta is mediated solely by selective estrogen receptor-α agonists and is abolished by estrogen deprivation. J Pharmacol Exp Ther 313: 1203–1208, 2005. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

