Molecular architecture of the yeast Mediator complex
- PMID: 26402457
- PMCID: PMC4631838
- DOI: 10.7554/eLife.08719
Molecular architecture of the yeast Mediator complex
Abstract
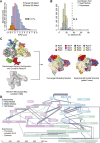
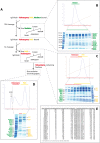
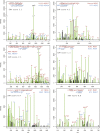
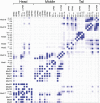
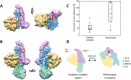
The 21-subunit Mediator complex transduces regulatory information from enhancers to promoters, and performs an essential role in the initiation of transcription in all eukaryotes. Structural information on two-thirds of the complex has been limited to coarse subunit mapping onto 2-D images from electron micrographs. We have performed chemical cross-linking and mass spectrometry, and combined the results with information from X-ray crystallography, homology modeling, and cryo-electron microscopy by an integrative modeling approach to determine a 3-D model of the entire Mediator complex. The approach is validated by the use of X-ray crystal structures as internal controls and by consistency with previous results from electron microscopy and yeast two-hybrid screens. The model shows the locations and orientations of all Mediator subunits, as well as subunit interfaces and some secondary structural elements. Segments of 20-40 amino acid residues are placed with an average precision of 20 Å. The model reveals roles of individual subunits in the organization of the complex.
Keywords: RNA polymerase II; S. cerevisiae; biophysics; cross-linking; mass spectrometry; modeling; structural biology; transcriptional regulation.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures









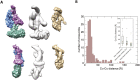
Similar articles
-
Core Mediator structure at 3.4 Å extends model of transcription initiation complex.Nature. 2017 May 11;545(7653):248-251. doi: 10.1038/nature22328. Epub 2017 May 3. Nature. 2017. PMID: 28467824
-
Structure of a Complete Mediator-RNA Polymerase II Pre-Initiation Complex.Cell. 2016 Sep 8;166(6):1411-1422.e16. doi: 10.1016/j.cell.2016.08.050. Cell. 2016. PMID: 27610567 Free PMC article.
-
Subunit architecture and functional modular rearrangements of the transcriptional mediator complex.Cell. 2014 Jun 5;157(6):1430-1444. doi: 10.1016/j.cell.2014.05.015. Epub 2014 May 29. Cell. 2014. PMID: 24882805 Free PMC article.
-
The complex structure and function of Mediator.J Biol Chem. 2018 Sep 7;293(36):13778-13785. doi: 10.1074/jbc.R117.794438. Epub 2017 Sep 14. J Biol Chem. 2018. PMID: 28912271 Free PMC article. Review.
-
Twenty years of Mediator complex structural studies.Biochem Soc Trans. 2019 Feb 28;47(1):399-410. doi: 10.1042/BST20180608. Epub 2019 Feb 7. Biochem Soc Trans. 2019. PMID: 30733343 Free PMC article. Review.
Cited by
-
The Evolving Contribution of Mass Spectrometry to Integrative Structural Biology.J Am Soc Mass Spectrom. 2016 Jun;27(6):966-74. doi: 10.1007/s13361-016-1382-4. Epub 2016 Apr 7. J Am Soc Mass Spectrom. 2016. PMID: 27056566 Free PMC article. Review.
-
PHYTOCHROME-INTERACTING FACTOR 4/HEMERA-mediated thermosensory growth requires the Mediator subunit MED14.Plant Physiol. 2022 Nov 28;190(4):2706-2721. doi: 10.1093/plphys/kiac412. Plant Physiol. 2022. PMID: 36063057 Free PMC article.
-
Systematic analysis of the expression profiles and prognostic significance of the MED gene family in renal clear cell carcinoma.Oncol Lett. 2024 Jun 26;28(2):398. doi: 10.3892/ol.2024.14531. eCollection 2024 Aug. Oncol Lett. 2024. PMID: 38979551 Free PMC article.
-
Auto-regulation of Rab5 GEF activity in Rabex5 by allosteric structural changes, catalytic core dynamics and ubiquitin binding.Elife. 2019 Nov 13;8:e46302. doi: 10.7554/eLife.46302. Elife. 2019. PMID: 31718772 Free PMC article.
-
Role of the pre-initiation complex in Mediator recruitment and dynamics.Elife. 2018 Dec 12;7:e39633. doi: 10.7554/eLife.39633. Elife. 2018. PMID: 30540252 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

