Correlations between Photodegradation of Bisretinoid Constituents of Retina and Dicarbonyl Adduct Deposition
- PMID: 26400086
- PMCID: PMC4646369
- DOI: 10.1074/jbc.M115.680363
Correlations between Photodegradation of Bisretinoid Constituents of Retina and Dicarbonyl Adduct Deposition
Abstract
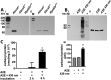
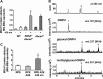
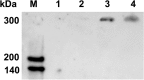
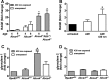
Non-enzymatic collagen cross-linking and carbonyl adduct deposition are features of Bruch's membrane aging in the eye, and disturbances in extracellular matrix turnover are considered to contribute to Bruch's membrane thickening. Because bisretinoid constituents of the lipofuscin of retinal pigment epithelial (RPE) cells are known to photodegrade to mixtures of aldehyde-bearing fragments and small dicarbonyls (glyoxal (GO) and methylglyoxal (MG)), we investigated RPE lipofuscin as a source of the reactive species that covalently modify protein side chains. Abca4(-/-) and Rdh8(-/-)/Abca4(-/-) mice that are models of accelerated bisretinoid formation were studied and pre-exposure of mice to 430 nm light enriched for dicarbonyl release by bisretinoid photodegradation. MG protein adducts were elevated in posterior eyecups of mutant mice, whereas carbonylation of an RPE-specific protein was observed in Abca4(-/-) but not in wild-type mice under the same conditions. Immunolabeling of cryostat-sectioned eyes harvested from Abca4(-/-) mice revealed that carbonyl adduct deposition in Bruch's membrane was accentuated. Cell-based assays corroborated these findings in mice. Moreover, the receptor for advanced glycation end products that recognizes MG and GO adducts and glyoxylase 1 that metabolizes MG and GO were up-regulated in Abca4(-/-) mice. Additionally, in acellular assays, peptides were cross-linked in the presence of A2E (adduct of two vitamin A aldehyde and ethanolamine) photodegradation products, and in a zymography assay, reaction of collagen IV with products of A2E photodegradation resulted in reduced cleavage by the matrix metalloproteinases MMP2 and MMP9. In conclusion, these mechanistic studies demonstrate a link between the photodegradation of RPE bisretinoid fluorophores and aging changes in underlying Bruch's membrane that can confer risk of age-related macular degeneration.
Keywords: aging; basement membrane; bisretinoid; dicarbonyl; eye; lipofuscin; photodegradation; retina; retinal degeneration; retinal pigment epithelium.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures



Similar articles
-
A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration.PLoS One. 2012;7(7):e41309. doi: 10.1371/journal.pone.0041309. Epub 2012 Jul 19. PLoS One. 2012. PMID: 22829938 Free PMC article.
-
Iron promotes oxidative cell death caused by bisretinoids of retina.Proc Natl Acad Sci U S A. 2018 May 8;115(19):4963-4968. doi: 10.1073/pnas.1722601115. Epub 2018 Apr 23. Proc Natl Acad Sci U S A. 2018. PMID: 29686088 Free PMC article.
-
A novel bisretinoid of retina is an adduct on glycerophosphoethanolamine.Invest Ophthalmol Vis Sci. 2011 Nov 25;52(12):9084-90. doi: 10.1167/iovs.11-8632. Invest Ophthalmol Vis Sci. 2011. PMID: 22039245 Free PMC article.
-
Bisretinoid Photodegradation Is Likely Not a Good Thing.Adv Exp Med Biol. 2018;1074:395-401. doi: 10.1007/978-3-319-75402-4_49. Adv Exp Med Biol. 2018. PMID: 29721969 Free PMC article. Review.
-
Lipofuscin and macular degeneration.Nutr Rev. 2003 Oct;61(10):342-6. doi: 10.1301/nr.2003.oct.342-346. Nutr Rev. 2003. PMID: 14604266 Review.
Cited by
-
Bisretinoids of the Retina: Photo-Oxidation, Iron-Catalyzed Oxidation, and Disease Consequences.Antioxidants (Basel). 2021 Aug 29;10(9):1382. doi: 10.3390/antiox10091382. Antioxidants (Basel). 2021. PMID: 34573014 Free PMC article. Review.
-
Vitamin A-aldehyde adducts: AMD risk and targeted therapeutics.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):4564-9. doi: 10.1073/pnas.1600474113. Epub 2016 Apr 11. Proc Natl Acad Sci U S A. 2016. PMID: 27071115 Free PMC article.
-
Water-Soluble Products of Photooxidative Destruction of the Bisretinoid A2E Cause Proteins Modification in the Dark.Int J Mol Sci. 2022 Jan 28;23(3):1534. doi: 10.3390/ijms23031534. Int J Mol Sci. 2022. PMID: 35163454 Free PMC article.
-
Chemiexcitation: Mammalian Photochemistry in the Dark†.Photochem Photobiol. 2023 Mar;99(2):251-276. doi: 10.1111/php.13781. Epub 2023 Feb 7. Photochem Photobiol. 2023. PMID: 36681894 Free PMC article. Review.
-
Isopropyl-phloroglucinol-DHA protects outer retinal cells against lethal dose of all-trans-retinal.J Cell Mol Med. 2020 May;24(9):5057-5069. doi: 10.1111/jcmm.15135. Epub 2020 Mar 25. J Cell Mol Med. 2020. PMID: 32212312 Free PMC article.
References
-
- Rabbani N., and Thornalley P. J. (2015) Dicarbonyl stress in cell and tissue dysfunction contributing to ageing and disease. Biochem. Biophys. Res. Commun. 458, 221–226 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

