Inhibition of ovulation by administration of estetrol in combination with drospirenone or levonorgestrel: Results of a phase II dose-finding pilot study
- PMID: 26394847
- PMCID: PMC4673580
- DOI: 10.3109/13625187.2015.1074675
Inhibition of ovulation by administration of estetrol in combination with drospirenone or levonorgestrel: Results of a phase II dose-finding pilot study
Abstract
Objectives: The aim of the study was to evaluate the efficacy of different dosages of estetrol (E4) combined with one of two progestins in suppressing the pituitary-ovarian axis and ovulation in healthy premenopausal women.
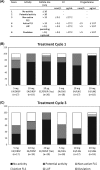
Methods: This was an open, parallel, phase II, dose-finding, pilot study performed in healthy women aged 18 to 35 years with a documented ovulatory cycle before treatment. For three consecutive cycles in a 24/4-day regimen, participants received 5 mg or 10 mg E4/3 mg drospirenone (DRSP); 5 mg, 10 mg or 20 mg E4/150 μg levonorgestrel; or 20 μg ethinylestradiol (EE)/3 mg DRSP as comparator. Pituitary-ovarian axis activity and the occurrence of ovulation were evaluated by monitoring follicular size, serum levels of follicle-stimulating hormone, luteinising hormone, estradiol and progesterone during treatment cycles 1 and 3. Endometrial thickness was evaluated throughout the trial, and the return of ovulation was evaluated after the last intake of medication.
Results: A total of 109 women were included in the trial. No ovulation occurred in any treatment group. Ovarian activity inhibition seemed proportional to the E4 dosage: the highest suppression was observed in the 20 mg E4 group and was very similar to that observed with EE/DRSP. Endometrial thickness was suppressed to the same extent in all groups. Post-treatment ovulation occurred in all participants between 17 and 21 days after the last active treatment. The study combinations were well tolerated and safe.
Conclusions: Combined with a progestin, E4 adequately suppresses ovarian activity, particularly when given at a dosage above 10 mg/day.
Keywords: Estetrol; Estrogen; Oral contraception; Ovulation inhibition; Progestin.
Figures



Similar articles
-
Effects of an oral contraceptive containing estetrol and drospirenone on ovarian function.Contraception. 2021 Jun;103(6):386-393. doi: 10.1016/j.contraception.2021.03.003. Epub 2021 Mar 6. Contraception. 2021. PMID: 33689786 Clinical Trial.
-
Unique effects on hepatic function, lipid metabolism, bone and growth endocrine parameters of estetrol in combined oral contraceptives.Eur J Contracept Reprod Health Care. 2015;20(6):463-75. doi: 10.3109/13625187.2015.1068934. Epub 2015 Jul 27. Eur J Contracept Reprod Health Care. 2015. PMID: 26212489 Free PMC article. Clinical Trial.
-
Evaluation of the effect of a new oral contraceptive containing estetrol and drospirenone on hemostasis parameters.Contraception. 2020 Dec;102(6):396-402. doi: 10.1016/j.contraception.2020.08.015. Epub 2020 Sep 19. Contraception. 2020. PMID: 32956694 Clinical Trial.
-
Pharmacodynamics of combined estrogen-progestin oral contraceptives 3. Inhibition of ovulation.Expert Rev Clin Pharmacol. 2018 Nov;11(11):1085-1098. doi: 10.1080/17512433.2018.1536544. Epub 2018 Nov 6. Expert Rev Clin Pharmacol. 2018. PMID: 30325245 Review.
-
Combined oral contraceptive with estetrol plus drospirenone: from pharmacokinetics to clinical applications.Expert Opin Drug Metab Toxicol. 2023 Dec;19(12):871-879. doi: 10.1080/17425255.2023.2279752. Epub 2024 Jan 12. Expert Opin Drug Metab Toxicol. 2023. PMID: 37942662 Review.
Cited by
-
Role of Membrane Estrogen Receptor Alpha on the Positive Feedback of Estrogens on Kisspeptin and GnRH Neurons.eNeuro. 2024 Oct 23;11(10):ENEURO.0271-23.2024. doi: 10.1523/ENEURO.0271-23.2024. Print 2024 Oct. eNeuro. 2024. PMID: 39375032 Free PMC article.
-
Estetrol/Drospirenone: A Review in Oral Contraception.Drugs. 2022 Jul;82(10):1117-1125. doi: 10.1007/s40265-022-01738-8. Epub 2022 Jul 4. Drugs. 2022. PMID: 35781795 Free PMC article. Review.
-
Systematic Review of Ovarian Activity and Potential for Embryo Formation and Loss during the Use of Hormonal Contraception.Linacre Q. 2018 Nov;85(4):453-469. doi: 10.1177/0024363918815611. Epub 2019 Jan 3. Linacre Q. 2018. PMID: 32431378 Free PMC article.
-
Estetrol and Mammary Gland: Friends or Foes?J Mammary Gland Biol Neoplasia. 2021 Sep;26(3):297-308. doi: 10.1007/s10911-021-09497-0. Epub 2021 Aug 31. J Mammary Gland Biol Neoplasia. 2021. PMID: 34463898 Free PMC article. Review.
-
Progestogen-only oral contraceptives.Rev Bras Ginecol Obstet. 2022 Apr;44(4):442-448. doi: 10.1055/s-0042-1748754. Epub 2022 May 27. Rev Bras Ginecol Obstet. 2022. PMID: 35623623 Free PMC article. No abstract available.
References
-
- Hagen AA, Barr M, Diczfalusy E. Metabolism of 17-beta-estradiol-4-14-C in Early Infancy. Acta Endocrinol. 1965;49:207–20. - PubMed
-
- Visser M, Coelingh Bennink HJ. Clinical applications for estetrol. J Steroid Biochem Mol Biol. 2009;114((1–2)):85–9. - PubMed
-
- Coelingh Bennink HJ, Skouby S, Bouchard P, et al. Ovulation inhibition by estetrol in an in vivo model. Contraception. 2008;77:186–90. - PubMed
-
- Cleuren AC, Van der Linden IK, De Visser YP, et al. 17alpha-ethinylestradiol rapidly alters transcript levels of murine coagulation genes via estrogen receptor alpha. J Thromb Haemost. 2010;8:1838–46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
