Tegument Assembly and Secondary Envelopment of Alphaherpesviruses
- PMID: 26393641
- PMCID: PMC4584305
- DOI: 10.3390/v7092861
Tegument Assembly and Secondary Envelopment of Alphaherpesviruses
Abstract
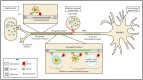
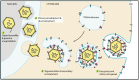
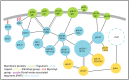
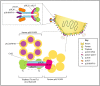
Alphaherpesviruses like herpes simplex virus are large DNA viruses characterized by their ability to establish lifelong latent infection in neurons. As for all herpesviruses, alphaherpesvirus virions contain a protein-rich layer called "tegument" that links the DNA-containing capsid to the glycoprotein-studded membrane envelope. Tegument proteins mediate a diverse range of functions during the virus lifecycle, including modulation of the host-cell environment immediately after entry, transport of virus capsids to the nucleus during infection, and wrapping of cytoplasmic capsids with membranes (secondary envelopment) during virion assembly. Eleven tegument proteins that are conserved across alphaherpesviruses have been implicated in the formation of the tegument layer or in secondary envelopment. Tegument is assembled via a dense network of interactions between tegument proteins, with the redundancy of these interactions making it challenging to determine the precise function of any specific tegument protein. However, recent studies have made great headway in defining the interactions between tegument proteins, conserved across alphaherpesviruses, which facilitate tegument assembly and secondary envelopment. We summarize these recent advances and review what remains to be learned about the molecular interactions required to assemble mature alphaherpesvirus virions following the release of capsids from infected cell nuclei.
Keywords: HSV-1; PrV; herpes simplex virus; pseudorabies virus; virus egress; virus maturation.
Figures
Similar articles
-
Conserved Tryptophan Motifs in the Large Tegument Protein pUL36 Are Required for Efficient Secondary Envelopment of Herpes Simplex Virus Capsids.J Virol. 2016 May 12;90(11):5368-5383. doi: 10.1128/JVI.03167-15. Print 2016 Jun 1. J Virol. 2016. PMID: 27009950 Free PMC article.
-
An ESCRT/VPS4 Envelopment Trap To Examine the Mechanism of Alphaherpesvirus Assembly and Transport in Neurons.J Virol. 2022 Mar 23;96(6):e0217821. doi: 10.1128/jvi.02178-21. Epub 2022 Jan 19. J Virol. 2022. PMID: 35045266 Free PMC article.
-
The UL48 tegument protein of pseudorabies virus is critical for intracytoplasmic assembly of infectious virions.J Virol. 2002 Jul;76(13):6729-42. doi: 10.1128/jvi.76.13.6729-6742.2002. J Virol. 2002. PMID: 12050386 Free PMC article.
-
Innate Immune Evasion of Alphaherpesvirus Tegument Proteins.Front Immunol. 2019 Sep 13;10:2196. doi: 10.3389/fimmu.2019.02196. eCollection 2019. Front Immunol. 2019. PMID: 31572398 Free PMC article. Review.
-
Comprehensive Analysis of the Tegument Proteins Involved in Capsid Transport and Virion Morphogenesis of Alpha, Beta and Gamma Herpesviruses.Viruses. 2023 Oct 6;15(10):2058. doi: 10.3390/v15102058. Viruses. 2023. PMID: 37896835 Free PMC article. Review.
Cited by
-
The incredible bulk: Human cytomegalovirus tegument architectures uncovered by AI-empowered cryo-EM.Sci Adv. 2024 Feb 23;10(8):eadj1640. doi: 10.1126/sciadv.adj1640. Epub 2024 Feb 23. Sci Adv. 2024. PMID: 38394211 Free PMC article.
-
LIVE-CELL FLUORESCENCE MICROSCOPY OF HSV-1 CELLULAR EGRESS BY EXOCYTOSIS.bioRxiv [Preprint]. 2023 Aug 17:2023.02.27.530373. doi: 10.1101/2023.02.27.530373. bioRxiv. 2023. PMID: 36909512 Free PMC article. Preprint.
-
Qualitative Differences in Capsidless L-Particles Released as a By-Product of Bovine Herpesvirus 1 and Herpes Simplex Virus 1 Infections.J Virol. 2018 Oct 29;92(22):e01259-18. doi: 10.1128/JVI.01259-18. Print 2018 Nov 15. J Virol. 2018. PMID: 30185590 Free PMC article.
-
DNA-Packing Portal and Capsid-Associated Tegument Complexes in the Tumor Herpesvirus KSHV.Cell. 2019 Sep 5;178(6):1329-1343.e12. doi: 10.1016/j.cell.2019.07.035. Epub 2019 Aug 22. Cell. 2019. PMID: 31447177 Free PMC article.
-
When liquid-liquid phase separation meets viral infections.Front Immunol. 2022 Aug 9;13:985622. doi: 10.3389/fimmu.2022.985622. eCollection 2022. Front Immunol. 2022. PMID: 36016945 Free PMC article. Review.
References
-
- Mocarski E.S., Jr. Comparative analysis of herpesvirus-common proteins. In: Arvin A., Campadelli-Fiume G., Mocarski E., Moore P.S., Roizman B., Whitley R., Yamanishi K., editors. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. pp. 44–58. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

