Effect of Amaranthus on Advanced Glycation End-Products Induced Cytotoxicity and Proinflammatory Cytokine Gene Expression in SH-SY5Y Cells
- PMID: 26393562
- PMCID: PMC6332459
- DOI: 10.3390/molecules200917288
Effect of Amaranthus on Advanced Glycation End-Products Induced Cytotoxicity and Proinflammatory Cytokine Gene Expression in SH-SY5Y Cells
Abstract
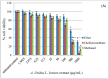
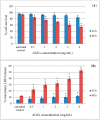
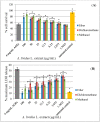
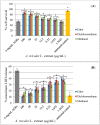
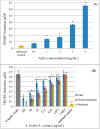
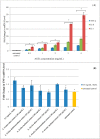
Amaranthus plants, or spinach, are used extensively as a vegetable and are known to possess medicinal properties. Neuroinflammation and oxidative stress play a major role in the pathogenesis of many neurodegenerative diseases, such as Alzheimer's disease and Parkinson's disease. Advanced glycation end-products (AGEs) cause cell toxicity in the human neuronal cell line, SH-SY5Y, through an increase in oxidative stress, as shown by reducing cell viability and increasing cell toxicity in a dose-dependent manner. We found that preincubation of SH-SY5Y cells with either petroleum ether, dichloromethane or methanol extracts of A. lividus and A. tricolor dose-dependently attenuated the neuron toxicity caused by AGEs treatment. Moreover, the results showed that A. lividus and A. tricolor extracts significantly downregulated the gene expression of the pro-inflammatory cytokines, TNF-α, IL-1 and IL-6 genes in AGEs-induced cells. We concluded that A. lividus and A. tricolor extracts not only have a neuroprotective effect against AGEs toxicity, but also have anti-inflammatory activity by reducing pro-inflammatory cytokine gene expression. This suggests that Amaranthus may be useful for treating chronic inflammation associated with neurodegenerative disorders.
Keywords: AGEs; Amaranthus; SH-SY5Y cells; oxidative stress; proinflammatory cytokines.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Neuroprotective effect of Amaranthus lividus and Amaranthus tricolor and their effects on gene expression of RAGE during oxidative stress in SH-SY5Y cells.Genet Mol Res. 2016 Apr 26;15(2). doi: 10.4238/gmr.15027562. Genet Mol Res. 2016. PMID: 27173239
-
The neuroprotective role of rosiglitazone in advanced glycation end product treated human neural stem cells is PPARgamma-dependent.Int J Biochem Cell Biol. 2017 Nov;92:121-133. doi: 10.1016/j.biocel.2017.09.020. Epub 2017 Sep 28. Int J Biochem Cell Biol. 2017. PMID: 28964868
-
Piper sarmentosum Roxb. confers neuroprotection on beta-amyloid (Aβ)-induced microglia-mediated neuroinflammation and attenuates tau hyperphosphorylation in SH-SY5Y cells.J Ethnopharmacol. 2018 May 10;217:187-194. doi: 10.1016/j.jep.2018.02.025. Epub 2018 Feb 17. J Ethnopharmacol. 2018. PMID: 29462698
-
Cardioprotective effect of Amaranthus tricolor extract in isoprenaline induced myocardial damage in ovariectomized rats.Biomed Pharmacother. 2018 Jul;103:1154-1162. doi: 10.1016/j.biopha.2018.04.151. Epub 2018 Apr 27. Biomed Pharmacother. 2018. PMID: 29715759
-
Multifunctional activities of green tea catechins in neuroprotection. Modulation of cell survival genes, iron-dependent oxidative stress and PKC signaling pathway.Neurosignals. 2005;14(1-2):46-60. doi: 10.1159/000085385. Neurosignals. 2005. PMID: 15956814 Review.
Cited by
-
Phytonutrients, Colorant Pigments, Phytochemicals, and Antioxidant Potential of Orphan Leafy Amaranthus Species.Molecules. 2022 May 2;27(9):2899. doi: 10.3390/molecules27092899. Molecules. 2022. PMID: 35566250 Free PMC article.
-
The Role of Advanced Glycation End-Product Levels Measured by Skin Autofluorescence in the Development of Mitral Annular Calcification.J Cardiovasc Dev Dis. 2023 Sep 20;10(9):406. doi: 10.3390/jcdd10090406. J Cardiovasc Dev Dis. 2023. PMID: 37754835 Free PMC article.
-
Betalains in Some Species of the Amaranthaceae Family: A Review.Antioxidants (Basel). 2018 Apr 4;7(4):53. doi: 10.3390/antiox7040053. Antioxidants (Basel). 2018. PMID: 29617324 Free PMC article. Review.
-
Colorant Pigments, Nutrients, Bioactive Components, and Antiradical Potential of Danta Leaves (Amaranthus lividus).Antioxidants (Basel). 2022 Jun 20;11(6):1206. doi: 10.3390/antiox11061206. Antioxidants (Basel). 2022. PMID: 35740102 Free PMC article.
-
Role of medicinal plants in inhibiting SARS-CoV-2 and in the management of post-COVID-19 complications.Phytomedicine. 2022 Apr;98:153930. doi: 10.1016/j.phymed.2022.153930. Epub 2022 Jan 5. Phytomedicine. 2022. PMID: 35114450 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

