Differential Roles of Phospholipase D Proteins in FcεRI-Mediated Signaling and Mast Cell Function
- PMID: 26392467
- PMCID: PMC4610851
- DOI: 10.4049/jimmunol.1500665
Differential Roles of Phospholipase D Proteins in FcεRI-Mediated Signaling and Mast Cell Function
Abstract
Phospholipase D (PLD) proteins are enzymes that catalyze the hydrolysis of phosphatidylcholine to generate an important signaling lipid, phosphatidic acid. Phosphatidic acid is a putative second messenger implicated in the regulation of vesicular trafficking and cytoskeletal reorganization. Previous studies using inhibitors and overexpression of PLD proteins indicate that PLD1 and PLD2 play positive roles in FcεRI-mediated signaling and mast cell function. We used mice deficient in PLD1, PLD2, or both to study the function of these enzymes in mast cells. In contrast to published studies, we found that PLD1 deficiency impaired FcεRI-mediated mast cell degranulation; however, PLD2 deficiency enhanced it. Biochemical analysis showed that PLD deficiency affected activation of the PI3K pathway and RhoA. Furthermore, our data indicated that, although PLD1 deficiency impaired F-actin disassembly, PLD2 deficiency enhanced microtubule formation. Together, our results suggested that PLD1 and PLD2, two proteins that catalyze the same enzymatic reaction, regulate different steps in mast cell degranulation.
Copyright © 2015 by The American Association of Immunologists, Inc.
Figures
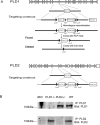
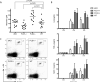
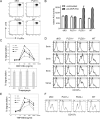
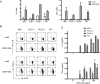

Similar articles
-
Continual production of phosphatidic acid by phospholipase D is essential for antigen-stimulated membrane ruffling in cultured mast cells.Mol Biol Cell. 2002 Oct;13(10):3730-46. doi: 10.1091/mbc.e02-04-0213. Mol Biol Cell. 2002. PMID: 12388770 Free PMC article.
-
Phospholipase D1 regulates high-affinity IgE receptor-induced mast cell degranulation.Blood. 2004 Dec 15;104(13):4122-8. doi: 10.1182/blood-2004-06-2091. Epub 2004 Aug 31. Blood. 2004. PMID: 15339843
-
SHIP down-regulates FcepsilonR1-induced degranulation at supraoptimal IgE or antigen levels.J Immunol. 2005 Jan 1;174(1):507-16. doi: 10.4049/jimmunol.174.1.507. J Immunol. 2005. PMID: 15611277
-
Signalling role for ARF and phospholipase D in mast cell exocytosis stimulated by crosslinking of the high affinity FcepsilonR1 receptor.Mol Immunol. 2002 Sep;38(16-18):1277-82. doi: 10.1016/s0161-5890(02)00075-5. Mol Immunol. 2002. PMID: 12217395 Review.
-
Amplification mechanisms for the enhancement of antigen-mediated mast cell activation.Immunol Res. 2009;43(1-3):15-24. doi: 10.1007/s12026-008-8046-9. Immunol Res. 2009. PMID: 18827981 Free PMC article. Review.
Cited by
-
Lipidomic analysis of urinary exosomes from hereditary α-tryptasemia patients and healthy volunteers.FASEB Bioadv. 2019 Oct;1(10):624-638. doi: 10.1096/fba.2019-00030. Epub 2019 Aug 24. FASEB Bioadv. 2019. PMID: 31803861 Free PMC article.
-
Altered Metabolism of Phospholipases, Diacylglycerols, Endocannabinoids, and N-Acylethanolamines in Patients with Mastocytosis.J Immunol Res. 2019 Jul 1;2019:5836476. doi: 10.1155/2019/5836476. eCollection 2019. J Immunol Res. 2019. PMID: 31355297 Free PMC article.
-
Phosphatidic Acid Stimulates Myoblast Proliferation through Interaction with LPA1 and LPA2 Receptors.Int J Mol Sci. 2021 Feb 1;22(3):1452. doi: 10.3390/ijms22031452. Int J Mol Sci. 2021. PMID: 33535610 Free PMC article.
-
Ethanol Inhibits High-Affinity Immunoglobulin E Receptor (FcεRI) Signaling in Mast Cells by Suppressing the Function of FcεRI-Cholesterol Signalosome.PLoS One. 2015 Dec 14;10(12):e0144596. doi: 10.1371/journal.pone.0144596. eCollection 2015. PLoS One. 2015. PMID: 26658290 Free PMC article.
-
Phospholipase D in TCR-Mediated Signaling and T Cell Activation.J Immunol. 2018 Mar 15;200(6):2165-2173. doi: 10.4049/jimmunol.1701291. Epub 2018 Jan 31. J Immunol. 2018. PMID: 29386256 Free PMC article.
References
-
- Siraganian RP. Mast cell signal transduction from the high-affinity IgE receptor. Curr Opin Immunol. 2003;15:639–646. - PubMed
-
- Rivera J. Molecular adapters in Fc(epsilon)RI signaling and the allergic response. Curr Opin Immunol. 2002;14:688–693. - PubMed
-
- Kinet JP. The high-affinity IgE receptor (Fc epsilon RI): from physiology to pathology. Annu Rev Immunol. 1999;17:931–972. - PubMed
-
- Brdicka T, Imrich M, Angelisova P, Brdickova N, Horvath O, Spicka J, Hilgert I, Luskova P, Draber P, Novak P, Engels N, Wienands J, Simeoni L, Osterreicher J, Aguado E, Malissen M, Schraven B, Horejsi V. Non-T cell activation linker (NTAL): a transmembrane adaptor protein involved in immunoreceptor signaling. J Exp Med. 2002;196:1617–1626. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

