Encapsulation of PEGylated low-molecular-weight PEI polyplexes in hyaluronic acid hydrogels reduces aggregation
- PMID: 26391497
- PMCID: PMC4648651
- DOI: 10.1016/j.actbio.2015.09.020
Encapsulation of PEGylated low-molecular-weight PEI polyplexes in hyaluronic acid hydrogels reduces aggregation
Abstract
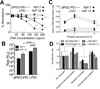
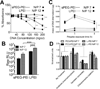
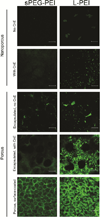
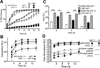
The effective delivery of DNA locally could increase the applicability of gene therapy in tissue regeneration and therapeutic angiogenesis. One promising approach is through use of porous hydrogel scaffolds that incorporate and deliver DNA in the form of nanoparticles to the affected sites. While we have previously reported on caged nanoparticle encapsulation (CnE) to load DNA polyplexes within hydrogels at high concentrations without aggregation, frequent issues with limited polyplex release following CnE have been encountered. In this study, we report two alternative approaches to polyplex presentation for decreasing aggregation in porous hydrogels. The first approach reduces polyplex aggregation by utilizing polyethylene glycol modification of the gene carrier polymer polyethyleneimine (sPEG-PEI) to mitigate charge-charge interactions between polyplexes and the scaffold during gelation. The second approach electrostatically presents polyplexes on the surfaces of scaffold pores as opposed to an encapsulated presentation. The sPEG-PEI polymer formed a smaller, less toxic, and more stable polyplex that exhibited less aggregation within HA gels when compared to the traditionally used linear PEI (LPEI) polymer. Surface-coated polyplexes also resulted in a more homogenous distribution of polyplexes in hydrogels. Furthermore, sPEG-PEI polyplexes retained transfection abilities comparable to LPEI in 3D surface-coated transfections. These results demonstrate a significant improvement in scaffold-mediated gene delivery and show promise in applications to multi-gene delivery systems.
Statement of significance: A promising gene delivery approach for regenerative medicine is implanting porous hydrogel scaffolds loaded with DNA nanoparticles for delivery to affected sites. However, loading DNA polyplexes at high concentrations within hydrogels results in significant aggregation. Here, we describe two methods for decreasing aggregation of DNA polyplexes in porous gels. First, the gene carrier polymer polyethyleneimine (PEI) was modified with polyethylene glycol (sPEG-PEI) to mitigate the electrostatic interactions between polyplexes and scaffold polymer to in turn decrease aggregation. Second, polyplexes were presented along the surfaces of the pores of the hydrogel instead of being encapsulated within the gel. These methods allow for highly tunable and sustained transgene expression from scaffold-mediated gene delivery while avoiding polyplex aggregation.
Keywords: Aggregation; Gene delivery; Hydrogel; Non-viral; PEGylation; Porous.
Copyright © 2015 Acta Materialia Inc. Published by Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Incorporation of active DNA/cationic polymer polyplexes into hydrogel scaffolds.Biomaterials. 2010 Dec;31(34):9106-16. doi: 10.1016/j.biomaterials.2010.08.016. Epub 2010 Sep 6. Biomaterials. 2010. PMID: 20822811 Free PMC article.
-
Hyaluronic acid and fibrin hydrogels with concentrated DNA/PEI polyplexes for local gene delivery.J Control Release. 2011 Aug 10;153(3):255-61. doi: 10.1016/j.jconrel.2011.01.028. Epub 2011 Feb 2. J Control Release. 2011. PMID: 21295089 Free PMC article.
-
Design and characterization of microporous hyaluronic acid hydrogels for in vitro gene transfer to mMSCs.Acta Biomater. 2012 Nov;8(11):3921-31. doi: 10.1016/j.actbio.2012.07.014. Epub 2012 Jul 20. Acta Biomater. 2012. PMID: 22820309 Free PMC article.
-
Polyethylenimine (PEI) in gene therapy: Current status and clinical applications.J Control Release. 2023 Oct;362:667-691. doi: 10.1016/j.jconrel.2023.09.001. Epub 2023 Sep 18. J Control Release. 2023. PMID: 37666302 Review.
-
Porous Alginate Scaffolds Assembled Using Vaterite CaCO3 Crystals.Micromachines (Basel). 2019 May 29;10(6):357. doi: 10.3390/mi10060357. Micromachines (Basel). 2019. PMID: 31146472 Free PMC article. Review.
Cited by
-
Hydrogel-Based Controlled Delivery Systems for Articular Cartilage Repair.Biomed Res Int. 2016;2016:1215263. doi: 10.1155/2016/1215263. Epub 2016 Aug 23. Biomed Res Int. 2016. PMID: 27642587 Free PMC article.
-
Hydrogel-Based Localized Nonviral Gene Delivery in Regenerative Medicine Approaches-An Overview.Pharmaceutics. 2020 Aug 10;12(8):752. doi: 10.3390/pharmaceutics12080752. Pharmaceutics. 2020. PMID: 32785171 Free PMC article. Review.
-
The biological applications of DNA nanomaterials: current challenges and future directions.Signal Transduct Target Ther. 2021 Oct 8;6(1):351. doi: 10.1038/s41392-021-00727-9. Signal Transduct Target Ther. 2021. PMID: 34620843 Free PMC article. Review.
-
Nucleic Acid Delivery from Granular Hydrogels.Adv Healthc Mater. 2022 Feb;11(3):e2101867. doi: 10.1002/adhm.202101867. Epub 2021 Nov 23. Adv Healthc Mater. 2022. PMID: 34742164 Free PMC article.
-
Pathways Governing Polyethylenimine Polyplex Transfection in Microporous Annealed Particle Scaffolds.Bioconjug Chem. 2019 Feb 20;30(2):476-486. doi: 10.1021/acs.bioconjchem.8b00696. Epub 2018 Dec 18. Bioconjug Chem. 2019. PMID: 30513197 Free PMC article.
References
-
- Lavik E, Langer R. Tissue engineering: current state and perspectives. Applied microbiology and biotechnology. 2004;65(1):1–8. - PubMed
-
- Nerem RM, Sambanis a. Tissue engineering: from biology to biological substitutes. Tissue engineering. 1995;1(1):3–13. - PubMed
-
- Lutolf MP, Hubbell Ja. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nature biotechnology. 2005;23(1):47–55. - PubMed
-
- Bonadio J, Smiley E, Patil P, Goldstein S. Localized, direct plasmid gene delivery in vivo: prolonged therapy results in reproducible tissue regeneration. Nature medicine. 1999;5(7):753–759. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

