Corruption of the dentate gyrus by "dominant" granule cells: Implications for dentate gyrus function in health and disease
- PMID: 26391451
- PMCID: PMC4792754
- DOI: 10.1016/j.nlm.2015.09.005
Corruption of the dentate gyrus by "dominant" granule cells: Implications for dentate gyrus function in health and disease
Abstract
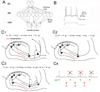
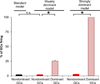
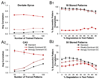
The dentate gyrus (DG) and area CA3 of the hippocampus are highly organized lamellar structures which have been implicated in specific cognitive functions such as pattern separation and pattern completion. Here we describe how the anatomical organization and physiology of the DG and CA3 are consistent with structures that perform pattern separation and completion. We then raise a new idea related to the complex circuitry of the DG and CA3 where CA3 pyramidal cell 'backprojections' play a potentially important role in the sparse firing of granule cells (GCs), considered important in pattern separation. We also propose that GC axons, the mossy fibers, already known for their highly specialized structure, have a dynamic function that imparts variance--'mossy fiber variance'--which is important to pattern separation and completion. Computational modeling is used to show that when a subset of GCs become 'dominant,' one consequence is loss of variance in the activity of mossy fiber axons and a reduction in pattern separation and completion in the model. Empirical data are then provided using an example of 'dominant' GCs--subsets of GCs that develop abnormally and have increased excitability. Notably, these abnormal GCs have been identified in animal models of disease where DG-dependent behaviors are impaired. Together these data provide insight into pattern separation and completion, and suggest that behavioral impairment could arise from dominance of a subset of GCs in the DG-CA3 network.
Keywords: Cognition; Computational modeling; Ectopic granule cells; Learning; Memory; Mossy fibers; Post-traumatic stress disorder (PTSD); Testosterone.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures


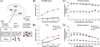


Similar articles
-
Dentate Gyrus Mossy Cells Share a Role in Pattern Separation with Dentate Granule Cells and Proximal CA3 Pyramidal Cells.J Neurosci. 2019 Nov 27;39(48):9570-9584. doi: 10.1523/JNEUROSCI.0940-19.2019. Epub 2019 Oct 22. J Neurosci. 2019. PMID: 31641051 Free PMC article.
-
Function of local circuits in the hippocampal dentate gyrus-CA3 system.Neurosci Res. 2019 Mar;140:43-52. doi: 10.1016/j.neures.2018.11.003. Epub 2018 Nov 5. Neurosci Res. 2019. PMID: 30408501 Review.
-
Pattern separation in the dentate gyrus: a role for the CA3 backprojection.Hippocampus. 2011 Nov;21(11):1190-215. doi: 10.1002/hipo.20828. Epub 2010 Aug 3. Hippocampus. 2011. PMID: 20683841 Free PMC article.
-
Impairment of Pattern Separation of Ambiguous Scenes by Single Units in the CA3 in the Absence of the Dentate Gyrus.J Neurosci. 2020 Apr 29;40(18):3576-3590. doi: 10.1523/JNEUROSCI.2596-19.2020. Epub 2020 Mar 31. J Neurosci. 2020. PMID: 32234778 Free PMC article.
-
Tracking the flow of hippocampal computation: Pattern separation, pattern completion, and attractor dynamics.Neurobiol Learn Mem. 2016 Mar;129:38-49. doi: 10.1016/j.nlm.2015.10.008. Epub 2015 Oct 26. Neurobiol Learn Mem. 2016. PMID: 26514299 Free PMC article. Review.
Cited by
-
AUTS2 Syndrome: Molecular Mechanisms and Model Systems.Front Mol Neurosci. 2022 Mar 31;15:858582. doi: 10.3389/fnmol.2022.858582. eCollection 2022. Front Mol Neurosci. 2022. PMID: 35431798 Free PMC article. Review.
-
Novel Psychopharmacological Herbs Relieve Behavioral Abnormalities and Hippocampal Dysfunctions in an Animal Model of Post-Traumatic Stress Disorder.Nutrients. 2023 Aug 31;15(17):3815. doi: 10.3390/nu15173815. Nutrients. 2023. PMID: 37686847 Free PMC article.
-
Gq-Coupled Muscarinic Receptor Enhancement of KCNQ2/3 Channels and Activation of TRPC Channels in Multimodal Control of Excitability in Dentate Gyrus Granule Cells.J Neurosci. 2019 Feb 27;39(9):1566-1587. doi: 10.1523/JNEUROSCI.1781-18.2018. Epub 2018 Dec 28. J Neurosci. 2019. PMID: 30593498 Free PMC article.
-
Population and individual firing behaviors in sparsely synchronized rhythms in the hippocampal dentate gyrus.Cogn Neurodyn. 2022 Jun;16(3):643-665. doi: 10.1007/s11571-021-09728-4. Epub 2021 Oct 23. Cogn Neurodyn. 2022. PMID: 35603046 Free PMC article.
-
Combination Antiretroviral Therapy (cART) in Diabetes Exacerbates Diabetogenic Effects on Hippocampal Microstructure, Neurogenesis and Cytokine Perturbation in Male Sprague Dawley Rats.Diagnostics (Basel). 2022 Apr 5;12(4):905. doi: 10.3390/diagnostics12040905. Diagnostics (Basel). 2022. PMID: 35453953 Free PMC article.
References
-
- Albert DJ, Walsh ML, Gorzalka BB, Siemens Y, Louie H. Testosterone removal in rats results in a decrease in social aggression and a loss of social dominance. Physiol Behav. 1986;36:401–407. - PubMed
-
- Alme CB, Buzzetti RA, Marrone DF, Leutgeb JK, Chawla MK, Schaner MJ, Bohanick JD, Khoboko T, Leutgeb S, Moser EI, Moser MB, McNaughton BL, Barnes CA. Hippocampal granule cells opt for early retirement. Hippocampus. 2010;20:1109–1123. - PubMed
-
- Amaral DG, Witter MP. The three-dimensional organization of the hippocampal formation: A review of anatomical data. Neuroscience. 1989;31:571–591. - PubMed
-
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. In: Storm-Mathisen J, Zimmer J, Ottersen OP, editors. Prog Brain Res. Vol. 83. Elsevier Science; 1990. pp. 1–11. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

