Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits
- PMID: 26390242
- PMCID: PMC4598295
- DOI: 10.1038/nm.3951
Critical role of acetylation in tau-mediated neurodegeneration and cognitive deficits
Abstract
Tauopathies, including frontotemporal dementia (FTD) and Alzheimer's disease (AD), are neurodegenerative diseases in which tau fibrils accumulate. Recent evidence supports soluble tau species as the major toxic species. How soluble tau accumulates and causes neurodegeneration remains unclear. Here we identify tau acetylation at Lys174 (K174) as an early change in AD brains and a critical determinant in tau homeostasis and toxicity in mice. The acetyl-mimicking mutant K174Q slows tau turnover and induces cognitive deficits in vivo. Acetyltransferase p300-induced tau acetylation is inhibited by salsalate and salicylate, which enhance tau turnover and reduce tau levels. In the PS19 transgenic mouse model of FTD, administration of salsalate after disease onset inhibited p300 activity, lowered levels of total tau and tau acetylated at K174, rescued tau-induced memory deficits and prevented hippocampal atrophy. The tau-lowering and protective effects of salsalate were diminished in neurons expressing K174Q tau. Targeting tau acetylation could be a new therapeutic strategy against human tauopathies.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
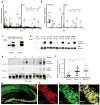
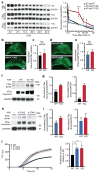
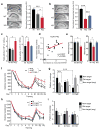
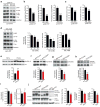
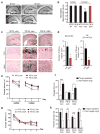
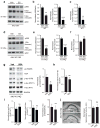
Comment in
-
Neurodegenerative disease: Targeting tau acetylation attenuates neurodegeneration.Nat Rev Drug Discov. 2015 Nov;14(11):748. doi: 10.1038/nrd4768. Nat Rev Drug Discov. 2015. PMID: 26514860 No abstract available.
Similar articles
-
Identification of retinoblastoma binding protein 7 (Rbbp7) as a mediator against tau acetylation and subsequent neuronal loss in Alzheimer's disease and related tauopathies.Acta Neuropathol. 2021 Aug;142(2):279-294. doi: 10.1007/s00401-021-02323-1. Epub 2021 May 12. Acta Neuropathol. 2021. PMID: 33978814 Free PMC article.
-
Anti-acetylated-tau immunotherapy is neuroprotective in tauopathy and brain injury.Mol Neurodegener. 2024 Jun 24;19(1):51. doi: 10.1186/s13024-024-00733-9. Mol Neurodegener. 2024. PMID: 38915105 Free PMC article.
-
SIRT1 Deacetylates Tau and Reduces Pathogenic Tau Spread in a Mouse Model of Tauopathy.J Neurosci. 2018 Apr 11;38(15):3680-3688. doi: 10.1523/JNEUROSCI.2369-17.2018. Epub 2018 Mar 14. J Neurosci. 2018. PMID: 29540553 Free PMC article.
-
Brief update on different roles of tau in neurodegeneration.IUBMB Life. 2011 Jul;63(7):495-502. doi: 10.1002/iub.467. IUBMB Life. 2011. PMID: 21698753 Review.
-
Tau alteration and neuronal degeneration in tauopathies: mechanisms and models.Biochim Biophys Acta. 2005 Jan 3;1739(2-3):331-54. doi: 10.1016/j.bbadis.2004.06.018. Biochim Biophys Acta. 2005. PMID: 15615650 Review.
Cited by
-
Epigallocatechin-3-gallate modulates Tau Post-translational modifications and cytoskeletal network.Oncotarget. 2021 May 25;12(11):1083-1099. doi: 10.18632/oncotarget.27963. eCollection 2021 May 25. Oncotarget. 2021. PMID: 34084282 Free PMC article.
-
Acetylated Tau Obstructs KIBRA-Mediated Signaling in Synaptic Plasticity and Promotes Tauopathy-Related Memory Loss.Neuron. 2016 Apr 20;90(2):245-60. doi: 10.1016/j.neuron.2016.03.005. Epub 2016 Mar 31. Neuron. 2016. PMID: 27041503 Free PMC article.
-
Sirtuins promote brain homeostasis, preventing Alzheimer's disease through targeting neuroinflammation.Front Physiol. 2022 Aug 15;13:962769. doi: 10.3389/fphys.2022.962769. eCollection 2022. Front Physiol. 2022. PMID: 36045741 Free PMC article. Review.
-
New Features about Tau Function and Dysfunction.Biomolecules. 2016 Apr 19;6(2):21. doi: 10.3390/biom6020021. Biomolecules. 2016. PMID: 27104579 Free PMC article. Review.
-
A soluble truncated tau species related to cognitive dysfunction and caspase-2 is elevated in the brain of Huntington's disease patients.Acta Neuropathol Commun. 2019 Jul 30;7(1):111. doi: 10.1186/s40478-019-0764-9. Acta Neuropathol Commun. 2019. PMID: 31358058 Free PMC article.
References
-
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol Berl. 1991;82:239–259. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 1R01AG036884/AG/NIA NIH HHS/United States
- P50 AG005131/AG/NIA NIH HHS/United States
- R01 AG036884/AG/NIA NIH HHS/United States
- RL1 NS062413/NS/NINDS NIH HHS/United States
- R01 NS059690/NS/NINDS NIH HHS/United States
- NS40251/NS/NINDS NIH HHS/United States
- R24 DK085610/DK/NIDDK NIH HHS/United States
- R01AG030207/AG/NIA NIH HHS/United States
- P30 NS065780/NS/NINDS NIH HHS/United States
- P30NS065780/NS/NINDS NIH HHS/United States
- R01 AG030207/AG/NIA NIH HHS/United States
- NS062413/NS/NINDS NIH HHS/United States
- P30 AI027763/AI/NIAID NIH HHS/United States
- P30 MH062512/MH/NIMH NIH HHS/United States
- R01 NS040251/NS/NINDS NIH HHS/United States
- S10 RR024615/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

