Slicer-independent mechanism drives small-RNA strand separation during human RISC assembly
- PMID: 26384428
- PMCID: PMC4627090
- DOI: 10.1093/nar/gkv937
Slicer-independent mechanism drives small-RNA strand separation during human RISC assembly
Abstract
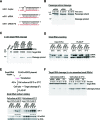
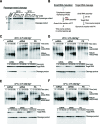
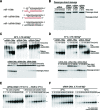
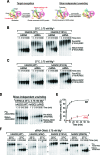
Small RNA silencing is mediated by the effector RNA-induced silencing complex (RISC) that consists of an Argonaute protein (AGOs 1-4 in humans). A fundamental step during RISC assembly involves the separation of two strands of a small RNA duplex, whereby only the guide strand is retained to form the mature RISC, a process not well understood. Despite the widely accepted view that 'slicer-dependent unwinding' via passenger-strand cleavage is a prerequisite for the assembly of a highly complementary siRNA into the AGO2-RISC, here we show by careful re-examination that 'slicer-independent unwinding' plays a more significant role in human RISC maturation than previously appreciated, not only for a miRNA duplex, but, unexpectedly, for a highly complementary siRNA as well. We discovered that 'slicer-dependency' for the unwinding was affected primarily by certain parameters such as temperature and Mg(2+). We further validate these observations in non-slicer AGOs (1, 3 and 4) that can be programmed with siRNAs at the physiological temperature of humans, suggesting that slicer-independent mechanism is likely a common feature of human AGOs. Our results now clearly explain why both miRNA and siRNA are found in all four human AGOs, which is in striking contrast to the strict small-RNA sorting system in Drosophila.
© The Author(s) 2015. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


Similar articles
-
The N domain of Argonaute drives duplex unwinding during RISC assembly.Nat Struct Mol Biol. 2012 Jan 10;19(2):145-51. doi: 10.1038/nsmb.2232. Nat Struct Mol Biol. 2012. PMID: 22233755
-
Slicer function of Drosophila Argonautes and its involvement in RISC formation.Genes Dev. 2005 Dec 1;19(23):2837-48. doi: 10.1101/gad.1370605. Epub 2005 Nov 14. Genes Dev. 2005. PMID: 16287716 Free PMC article.
-
Conversion of pre-RISC to holo-RISC by Ago2 during assembly of RNAi complexes.RNA. 2007 Jan;13(1):22-9. doi: 10.1261/rna.283207. Epub 2006 Nov 22. RNA. 2007. PMID: 17123955 Free PMC article.
-
Anatomy of RISC: how do small RNAs and chaperones activate Argonaute proteins?Wiley Interdiscip Rev RNA. 2016 Sep;7(5):637-60. doi: 10.1002/wrna.1356. Epub 2016 May 16. Wiley Interdiscip Rev RNA. 2016. PMID: 27184117 Free PMC article. Review.
-
[Components and assembly of RNA-induced silencing complex].Yi Chuan. 2006 Jun;28(6):761-6. Yi Chuan. 2006. PMID: 16818443 Review. Chinese.
Cited by
-
Analysis of AgoshRNA maturation and loading into Ago2.PLoS One. 2017 Aug 15;12(8):e0183269. doi: 10.1371/journal.pone.0183269. eCollection 2017. PLoS One. 2017. PMID: 28809941 Free PMC article.
-
Site-Specific Modification Using the 2'-Methoxyethyl Group Improves the Specificity and Activity of siRNAs.Mol Ther Nucleic Acids. 2017 Dec 15;9:242-250. doi: 10.1016/j.omtn.2017.10.003. Epub 2017 Oct 7. Mol Ther Nucleic Acids. 2017. PMID: 29246303 Free PMC article.
-
The Slicer Activity of ARGONAUTE1 Is Required Specifically for the Phasing, Not Production, of Trans-Acting Short Interfering RNAs in Arabidopsis.Plant Cell. 2016 Jul;28(7):1563-80. doi: 10.1105/tpc.16.00121. Epub 2016 Jun 27. Plant Cell. 2016. PMID: 27354557 Free PMC article.
-
Differences in silencing of mismatched targets by sliced versus diced siRNAs.Nucleic Acids Res. 2018 Jul 27;46(13):6806-6822. doi: 10.1093/nar/gky287. Nucleic Acids Res. 2018. PMID: 29718312 Free PMC article.
-
Regulation of microRNA biogenesis and its crosstalk with other cellular pathways.Nat Rev Mol Cell Biol. 2019 Jan;20(1):5-20. doi: 10.1038/s41580-018-0059-1. Nat Rev Mol Cell Biol. 2019. PMID: 30228348 Review.
References
-
- Hutvagner G., Simard M.J. Argonaute proteins: key players in RNA silencing. Nat. Rev. Mol. Cell Biol. 2008;9:22–32. - PubMed
-
- Kawamata T., Tomari Y. Making RISC. Trends Biochem. Sci. 2010;35:368–376. - PubMed
-
- Iki T., Yoshikawa M., Nishikiori M., Jaudal M.C., Matsumoto-Yokoyama E., Mitsuhara I., Meshi T., Ishikawa M. In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell. 2010;39:282–291. - PubMed
-
- Iwasaki S., Kobayashi M., Yoda M., Sakaguchi Y., Katsuma S., Suzuki T., Tomari Y. Hsc70/Hsp90 chaperone machinery mediates ATP-dependent RISC loading of small RNA duplexes. Mol. Cell. 2010;39:292–299. - PubMed
-
- Miyoshi T., Takeuchi A., Siomi H., Siomi M.C. A direct role for Hsp90 in pre-RISC formation in Drosophila. Nat. Struct. Mol. Biol. 2010;17:1024–1026. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

