Gene expression parallels synaptic excitability and plasticity changes in Alzheimer's disease
- PMID: 26379494
- PMCID: PMC4548151
- DOI: 10.3389/fncel.2015.00318
Gene expression parallels synaptic excitability and plasticity changes in Alzheimer's disease
Abstract
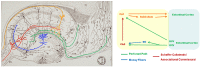
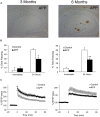
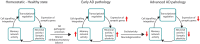
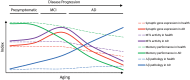
Alzheimer's disease (AD) is a neurodegenerative disorder characterized by abnormal accumulation of β-amyloid and tau and synapse dysfunction in memory-related neural circuits. Pathological and functional changes in the medial temporal lobe, a region essential for explicit memory encoding, contribute to cognitive decline in AD. Surprisingly, functional imaging studies show increased activity of the hippocampus and associated cortical regions during memory tasks in presymptomatic and early AD stages, whereas brain activity declines as the disease progresses. These findings suggest an emerging scenario where early pathogenic events might increase neuronal excitability leading to enhanced brain activity before clinical manifestations of the disease, a stage that is followed by decreased brain activity as neurodegeneration progresses. The mechanisms linking pathology with synaptic excitability and plasticity changes leading to memory loss in AD remain largely unclear. Recent studies suggest that increased brain activity parallels enhanced expression of genes involved in synaptic transmission and plasticity in preclinical stages, whereas expression of synaptic and activity-dependent genes are reduced by the onset of pathological and cognitive symptoms. Here, we review recent evidences indicating a relationship between transcriptional deregulation of synaptic genes and neuronal activity and memory loss in AD and mouse models. These findings provide the basis for potential clinical applications of memory-related transcriptional programs and their regulatory mechanisms as novel biomarkers and therapeutic targets to restore brain function in AD and other cognitive disorders.
Keywords: Alzheimer’s disease; Aβ; gene expression; memory; neurodegeneration; transcriptome.
Figures
Similar articles
-
Systematic characterization of a non-transgenic Aβ1-42 amyloidosis model: synaptic plasticity and memory deficits in female and male mice.Biol Sex Differ. 2023 Sep 16;14(1):59. doi: 10.1186/s13293-023-00545-4. Biol Sex Differ. 2023. PMID: 37716988 Free PMC article.
-
Crtc1 activates a transcriptional program deregulated at early Alzheimer's disease-related stages.J Neurosci. 2014 Apr 23;34(17):5776-87. doi: 10.1523/JNEUROSCI.5288-13.2014. J Neurosci. 2014. PMID: 24760838 Free PMC article.
-
Heightened Hippocampal β-Adrenergic Receptor Function Drives Synaptic Potentiation and Supports Learning and Memory in the TgF344-AD Rat Model during Prodromal Alzheimer's Disease.J Neurosci. 2021 Jun 30;41(26):5747-5761. doi: 10.1523/JNEUROSCI.0119-21.2021. Epub 2021 May 5. J Neurosci. 2021. PMID: 33952633 Free PMC article.
-
Synaptic Plasticity, Dementia and Alzheimer Disease.CNS Neurol Disord Drug Targets. 2017;16(3):220-233. doi: 10.2174/1871527316666170113120853. CNS Neurol Disord Drug Targets. 2017. PMID: 28088900 Review.
-
Mechanism of Oxidative Stress and Synapse Dysfunction in the Pathogenesis of Alzheimer's Disease: Understanding the Therapeutics Strategies.Mol Neurobiol. 2016 Jan;53(1):648-661. doi: 10.1007/s12035-014-9053-6. Epub 2014 Dec 17. Mol Neurobiol. 2016. PMID: 25511446 Free PMC article. Review.
Cited by
-
Spatial Memory Training Counteracts Hippocampal GIRK Channel Decrease in the Transgenic APPSw,Ind J9 Alzheimer's Disease Mouse Model.Int J Mol Sci. 2022 Nov 3;23(21):13444. doi: 10.3390/ijms232113444. Int J Mol Sci. 2022. PMID: 36362230 Free PMC article.
-
GenEpi: gene-based epistasis discovery using machine learning.BMC Bioinformatics. 2020 Feb 24;21(1):68. doi: 10.1186/s12859-020-3368-2. BMC Bioinformatics. 2020. PMID: 32093643 Free PMC article.
-
Transcriptomic changes in the prefrontal cortex of rats as a function of age and cognitive engagement.Neurobiol Learn Mem. 2019 Sep;163:107035. doi: 10.1016/j.nlm.2019.107035. Epub 2019 Jun 8. Neurobiol Learn Mem. 2019. PMID: 31185277 Free PMC article.
-
Perinatal Brain Docosahexaenoic Acid Concentration Has a Lasting Impact on Cognition in Mice.J Nutr. 2017 Sep;147(9):1624-1630. doi: 10.3945/jn.117.254607. Epub 2017 Aug 2. J Nutr. 2017. PMID: 28768838 Free PMC article.
-
Identification of Therapeutic Targets for Amyotrophic Lateral Sclerosis Using PandaOmics - An AI-Enabled Biological Target Discovery Platform.Front Aging Neurosci. 2022 Jun 28;14:914017. doi: 10.3389/fnagi.2022.914017. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35837482 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

