Interleukin-36γ is expressed by neutrophils and can activate microglia, but has no role in experimental autoimmune encephalomyelitis
- PMID: 26377915
- PMCID: PMC4574267
- DOI: 10.1186/s12974-015-0392-7
Interleukin-36γ is expressed by neutrophils and can activate microglia, but has no role in experimental autoimmune encephalomyelitis
Abstract
Background: Experimental autoimmune encephalomyelitis (EAE) is a model of inflammatory demyelinating diseases mediated by different types of leukocytes. How these cells communicate with each other to orchestrate autoimmune attacks is not fully understood, especially in the case of neutrophils, whose importance in EAE is newly established. The present study aimed to determine the expression pattern and role of different components of the IL-36 signaling pathway (IL-36α, IL-36β, IL-36γ, IL-36R) in EAE.
Methods: EAE was induced by either active immunization with myelin peptide, passive transfer of myelin-reactive T cells or injection of pertussis toxin to transgenic 2D2 mice. The molecules of interest were analyzed using a combination of techniques, including quantitative real-time PCR (qRT-PCR), flow cytometry, Western blotting, in situ hybridization, and immunohistochemistry. Microglial cultures were treated with recombinant IL-36γ and analyzed using DNA microarrays. Different mouse strains were subjected to clinical evaluation and flow cytometric analysis in order to compare their susceptibility to EAE.
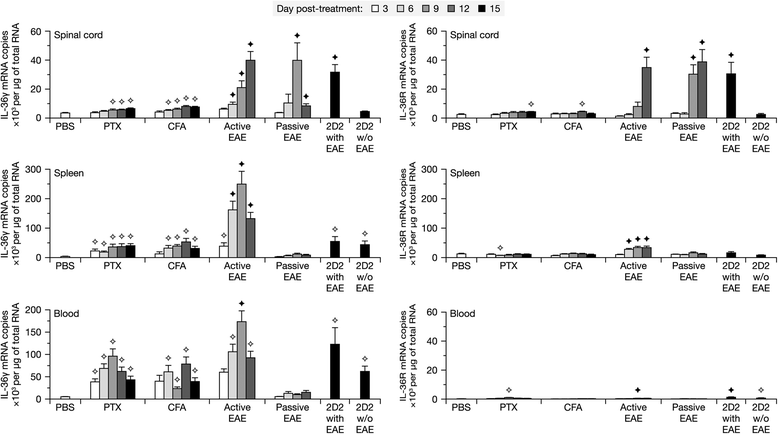
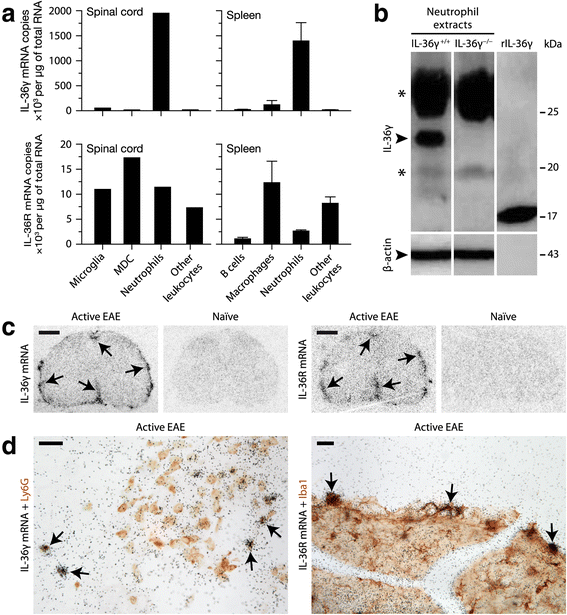
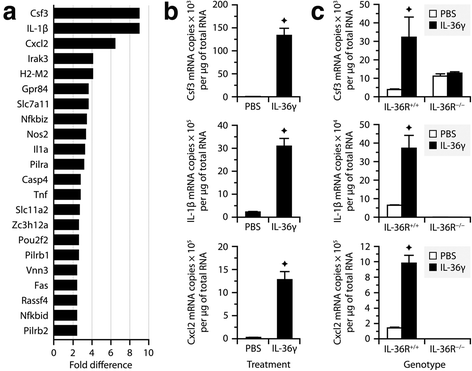
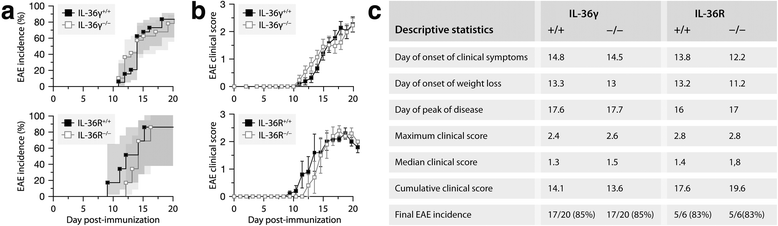
Results: Our observations indicate that both IL-36γ and IL-36R are strongly upregulated in nervous and hematopoietic tissues in different forms of EAE. IL-36γ is specifically expressed by neutrophils, while IL-36R is expressed by different immune cells, including microglia and other myeloid cells. In culture, microglia respond to recombinant IL-36γ by expressing molecules involved in neutrophil recruitment, such as Csf3, IL-1β, and Cxcl2. However, mice deficient in either IL-36γ or IL-36R develop similar clinical and histopathological signs of EAE compared to wild-type controls.
Conclusion: This study identifies IL-36γ as a neutrophil-related cytokine that can potentially activate microglia, but that is only correlative and not contributory in EAE.
Figures
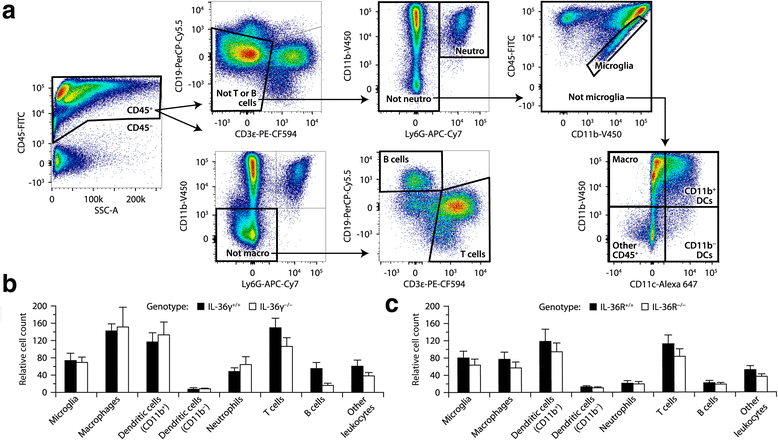
Similar articles
-
An IFNγ/CXCL2 regulatory pathway determines lesion localization during EAE.J Neuroinflammation. 2018 Jul 16;15(1):208. doi: 10.1186/s12974-018-1237-y. J Neuroinflammation. 2018. PMID: 30012158 Free PMC article.
-
Dual roles of the adenosine A2a receptor in autoimmune neuroinflammation.J Neuroinflammation. 2016 Feb 26;13:48. doi: 10.1186/s12974-016-0512-z. J Neuroinflammation. 2016. PMID: 26920550 Free PMC article.
-
Functional interleukin-17 receptor A is expressed in central nervous system glia and upregulated in experimental autoimmune encephalomyelitis.J Neuroinflammation. 2009 Apr 28;6:14. doi: 10.1186/1742-2094-6-14. J Neuroinflammation. 2009. PMID: 19400960 Free PMC article.
-
New Insights into the Role of IL-1β in Experimental Autoimmune Encephalomyelitis and Multiple Sclerosis.J Immunol. 2017 Jun 15;198(12):4553-4560. doi: 10.4049/jimmunol.1700263. J Immunol. 2017. PMID: 28583987 Free PMC article. Review.
-
Emerging Role of the IL-36/IL-36R Axis in Multiple Inflammatory Skin Diseases.J Invest Dermatol. 2024 Feb;144(2):206-224. doi: 10.1016/j.jid.2023.11.004. Epub 2024 Jan 7. J Invest Dermatol. 2024. PMID: 38189700 Review.
Cited by
-
Neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as prognostic predictors for delirium in critically ill patients: a systematic review and meta-analysis.BMC Anesthesiol. 2023 Feb 21;23(1):58. doi: 10.1186/s12871-023-01997-2. BMC Anesthesiol. 2023. PMID: 36803215 Free PMC article.
-
Costimulation Endows Immunotherapeutic CD8 T Cells with IL-36 Responsiveness during Aerobic Glycolysis.J Immunol. 2016 Jan 1;196(1):124-34. doi: 10.4049/jimmunol.1501217. Epub 2015 Nov 16. J Immunol. 2016. PMID: 26573834 Free PMC article.
-
IL-1 Family Members Mediate Cell Death, Inflammation and Angiogenesis in Retinal Degenerative Diseases.Front Immunol. 2019 Jul 16;10:1618. doi: 10.3389/fimmu.2019.01618. eCollection 2019. Front Immunol. 2019. PMID: 31379825 Free PMC article. Review.
-
Interleukin-36: Structure, Signaling and Function.Adv Exp Med Biol. 2021;21:191-210. doi: 10.1007/5584_2020_488. Adv Exp Med Biol. 2021. PMID: 32026417 Review.
-
IL-36 receptor deletion attenuates lung injury and decreases mortality in murine influenza pneumonia.Mucosal Immunol. 2017 Jul;10(4):1043-1055. doi: 10.1038/mi.2016.107. Epub 2016 Dec 14. Mucosal Immunol. 2017. PMID: 27966554 Free PMC article.
References
-
- Serafini B, Rosicarelli B, Magliozzi R, Stigliano E, Capello E, Mancardi GL, et al. Dendritic cells in multiple sclerosis lesions: maturation stage, myelin uptake, and interaction with proliferating T cells. J Neuropathol Exp Neurol. 2006;65:124–41. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

