Immune profile of an atypical EAE model in marmoset monkeys immunized with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund's adjuvant
- PMID: 26377397
- PMCID: PMC4574133
- DOI: 10.1186/s12974-015-0378-5
Immune profile of an atypical EAE model in marmoset monkeys immunized with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund's adjuvant
Abstract
Background: Experimental autoimmune encephalomyelitis (EAE) in the common marmoset monkey (Callithrix jacchus) is a relevant preclinical model for translational research into immunopathogenic mechanisms operating in multiple sclerosis (MS). Prior studies showed a core pathogenic role of T and B cells specific for myelin oligodendrocyte glycoprotein (MOG). However, in those studies, the quality of the response against MOG epitopes was strongly biased by bacterial antigens in the complete Freund's adjuvant (CFA), in which the immunizing recombinant human (rh) MOG protein had been formulated. In response to the need of a more refined EAE model, we have tested whether disease could also be induced with rhMOG in incomplete Freund's adjuvant (IFA).
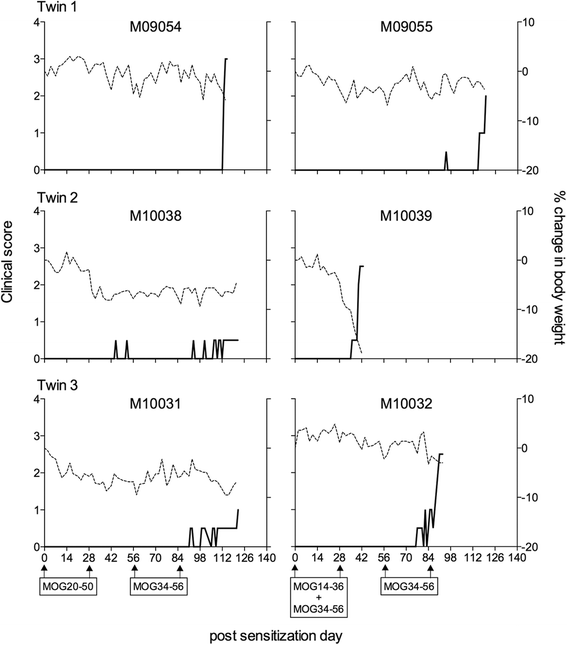
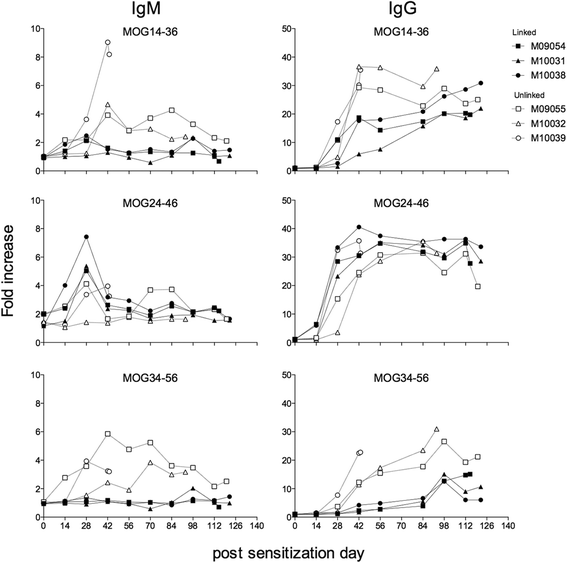
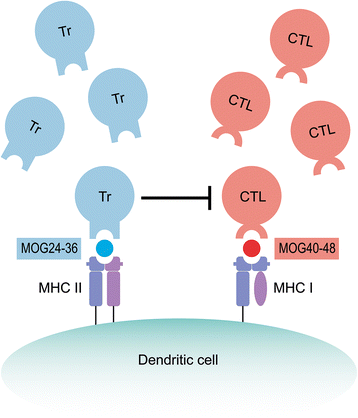
Method: Marmosets were immunized with rhMOG emulsified in IFA in the dorsal skin. Monkeys that did not develop neurological deficit were given booster immunizations at 28-day interval with the same antigen preparation. In a second experiment, three marmoset twin pairs were sensitized against MOG peptides in IFA to study a possibility for suppressive activity towards pathogenic T cells directed against the encephalitogenic epitope MOG40-48.
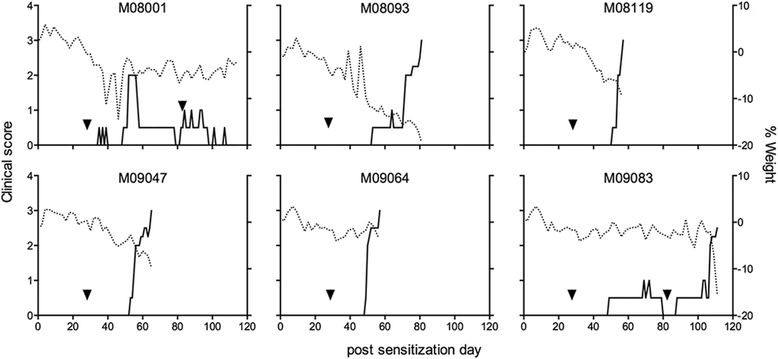
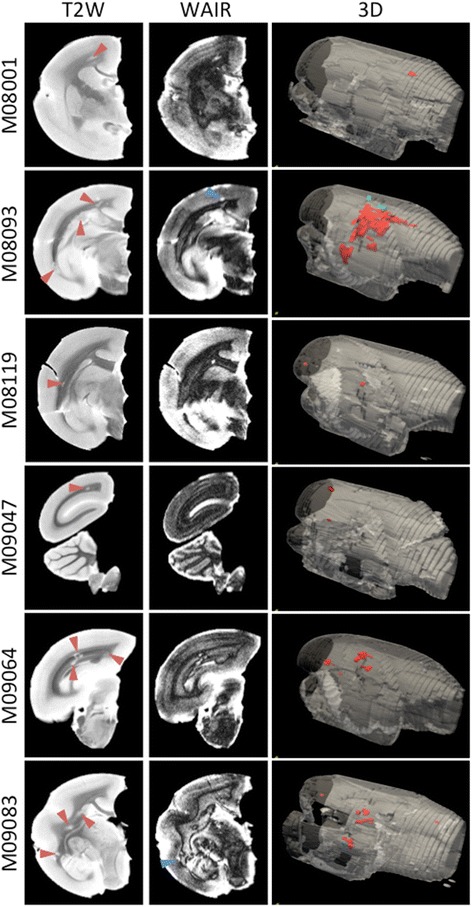
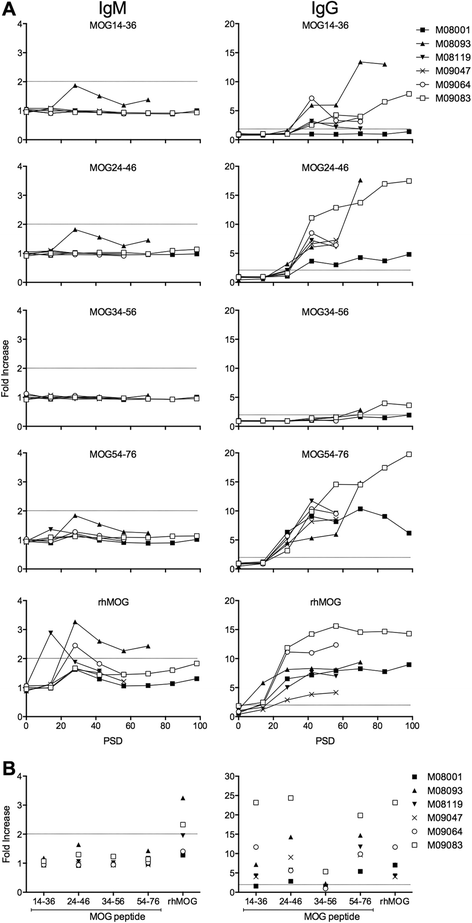
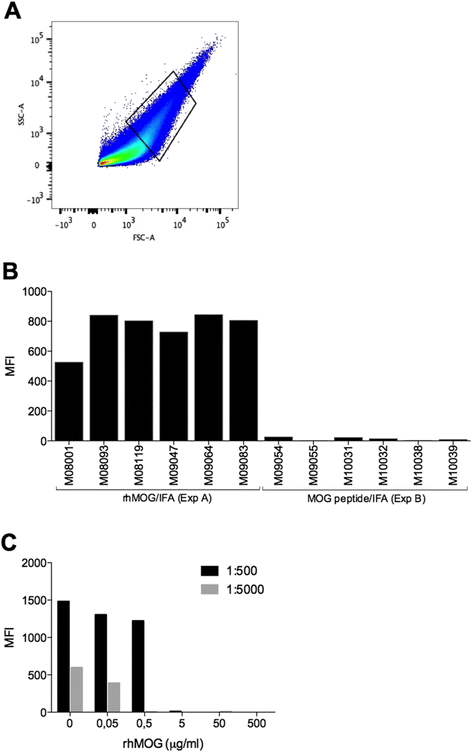
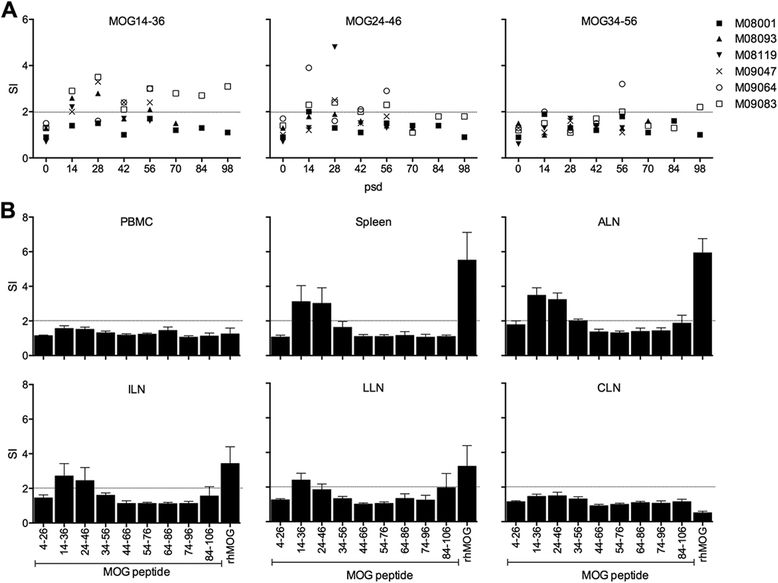
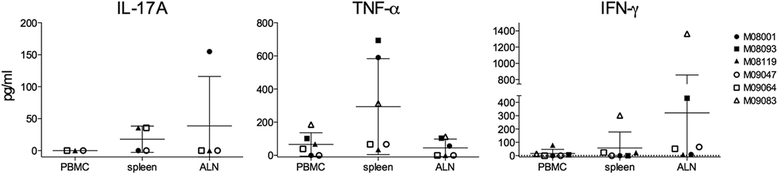
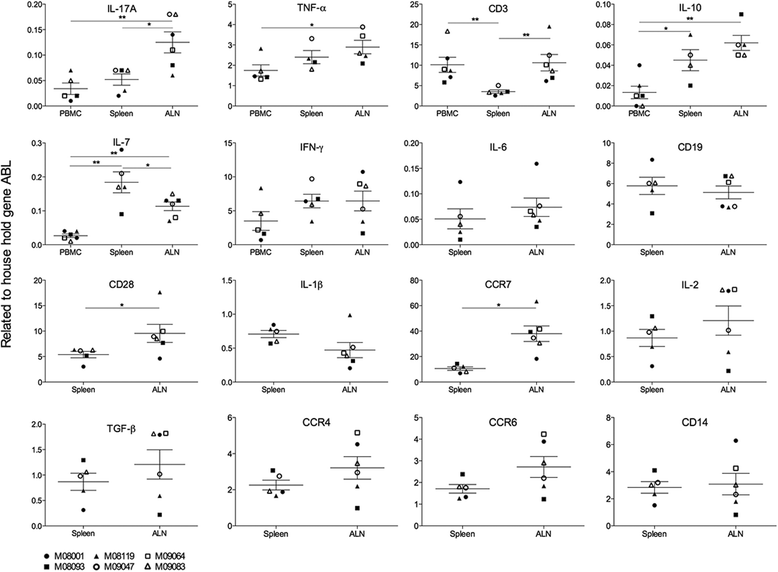
Results: Despite the absence of strong danger signals in the rhMOG/IFA inoculum, all monkeys developed clinically evident EAE symptoms. Moreover, in all monkeys, demyelinated lesions were present in the white matter and in two cases also in the cortical grey matter. Immune profiling at height of the disease showed a dominant T cell response against the overlapping peptides 14-36 and 24-46, but reactivity against the pathogenically most relevant peptide 34-56 was conspicuously absent. In the second experiment, there was an indication for a possible suppressive mechanism.
Conclusions: Immunization of marmoset monkeys with rhMOG in IFA elicits clinical EAE in all animals. Moreover, rhMOG contains pathogenic and regulatory epitopes, but the pathogenic hierarchy of rhMOG epitopes is strongly influenced by the adjuvant in which the protein is formulated.
Figures
Similar articles
-
A B Cell-Driven Autoimmune Pathway Leading to Pathological Hallmarks of Progressive Multiple Sclerosis in the Marmoset Experimental Autoimmune Encephalomyelitis Model.Front Immunol. 2017 Jul 11;8:804. doi: 10.3389/fimmu.2017.00804. eCollection 2017. Front Immunol. 2017. PMID: 28744286 Free PMC article. Review.
-
Experimental Autoimmune Encephalomyelitis (EAE) Model of Cynomolgus Macaques Induced by Recombinant Human MOG1-125 (rhMOG1-125) Protein and MOG34-56 Peptide.Protein Pept Lett. 2018 Feb 8;24(12):1166-1178. doi: 10.2174/0929866524666171110093626. Protein Pept Lett. 2018. PMID: 29141529
-
Induction of experimental autoimmune encephalomyelitis with recombinant human myelin oligodendrocyte glycoprotein in incomplete Freund's adjuvant in three non-human primate species.J Neuroimmune Pharmacol. 2013 Dec;8(5):1251-64. doi: 10.1007/s11481-013-9487-z. Epub 2013 Jul 3. J Neuroimmune Pharmacol. 2013. PMID: 23821341 Free PMC article.
-
T cell-depleted splenocytes from mice pre-immunized with neuroantigen in incomplete Freund's adjuvant involved in protection from experimental autoimmune encephalomyelitis.Immunol Lett. 2014 Jan-Feb;157(1-2):38-44. doi: 10.1016/j.imlet.2013.11.001. Epub 2013 Nov 9. Immunol Lett. 2014. PMID: 24220208
-
Experimental allergic encephalomyelitis in the New World monkey Callithrix jacchus.Immunol Rev. 2001 Oct;183:159-72. doi: 10.1034/j.1600-065x.2001.1830113.x. Immunol Rev. 2001. PMID: 11782255 Review.
Cited by
-
Dabigatran Suppresses PAR-1/SphK/S1P Activation of Astrocytes in Experimental Autoimmune Encephalomyelitis Model.Front Mol Neurosci. 2020 Jun 30;13:114. doi: 10.3389/fnmol.2020.00114. eCollection 2020. Front Mol Neurosci. 2020. PMID: 32694981 Free PMC article.
-
Severe oxidative stress in an acute inflammatory demyelinating model in the rhesus monkey.PLoS One. 2017 Nov 14;12(11):e0188013. doi: 10.1371/journal.pone.0188013. eCollection 2017. PLoS One. 2017. PMID: 29136024 Free PMC article.
-
A Tolerogenic Role of Cathepsin G in a Primate Model of Multiple Sclerosis: Abrogation by Epstein-Barr Virus Infection.Arch Immunol Ther Exp (Warsz). 2020 Jun 16;68(4):21. doi: 10.1007/s00005-020-00587-1. Arch Immunol Ther Exp (Warsz). 2020. PMID: 32556812 Free PMC article. Review.
-
A B Cell-Driven Autoimmune Pathway Leading to Pathological Hallmarks of Progressive Multiple Sclerosis in the Marmoset Experimental Autoimmune Encephalomyelitis Model.Front Immunol. 2017 Jul 11;8:804. doi: 10.3389/fimmu.2017.00804. eCollection 2017. Front Immunol. 2017. PMID: 28744286 Free PMC article. Review.
-
An Evaluation of 20 Years of EU Framework Programme-Funded Immune-Mediated Inflammatory Translational Research in Non-Human Primates.Front Immunol. 2016 Nov 7;7:462. doi: 10.3389/fimmu.2016.00462. eCollection 2016. Front Immunol. 2016. PMID: 27872622 Free PMC article.
References
-
- ‘t Hart BA, Bauer J, Muller HJ, Melchers B, Nicolay K, Brok H, et al. Histopathological characterization of magnetic resonance imaging-detectable brain white matter lesions in a primate model of multiple sclerosis: a correlative study in the experimental autoimmune encephalomyelitis model in common marmosets (Callithrix jacchus) Am J Pathol. 1998;153(2):649–63. doi: 10.1016/S0002-9440(10)65606-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

