Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice
- PMID: 26376849
- PMCID: PMC4574263
- DOI: 10.1186/s12931-015-0271-x
Vitamin D deficiency exacerbates COPD-like characteristics in the lungs of cigarette smoke-exposed mice
Abstract
Background: Chronic obstructive pulmonary disease (COPD) is characterized by excessive inflammation and disturbed bacterial clearance in the airways. Although cigarette smoke (CS) exposure poses a major risk, vitamin D deficiency could potentially contribute to COPD progression. Many in vitro studies demonstrate important anti-inflammatory and antibacterial effects of vitamin D, but a direct contribution of vitamin D deficiency to COPD onset and disease progression has not been explored.
Methods: In the current study, we used a murine experimental model to investigate the combined effect of vitamin D deficiency and CS exposure on the development of COPD-like characteristics. Therefore, vitamin D deficient or control mice were exposed to CS or ambient air for a period of 6 (subacute) or 12 weeks (chronic). Besides lung function and structure measurements, we performed an in depth analysis of the size and composition of the cellular infiltrate in the airways and lung parenchyma and tested the ex vivo phagocytic and oxidative burst capacity of alveolar macrophages.
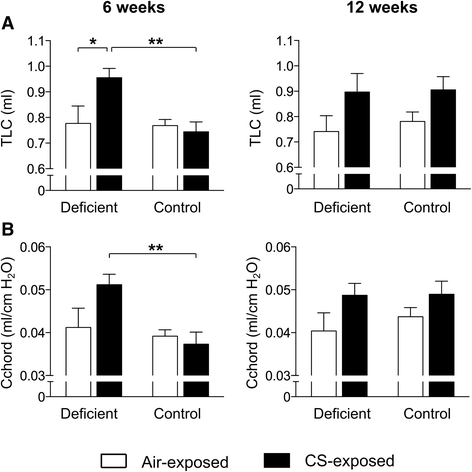
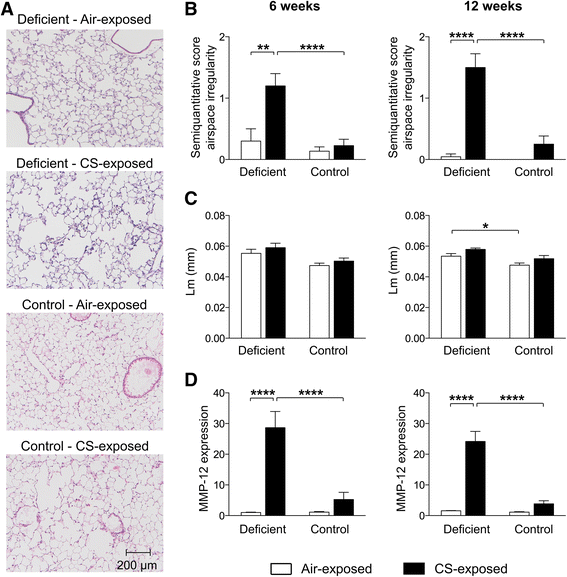
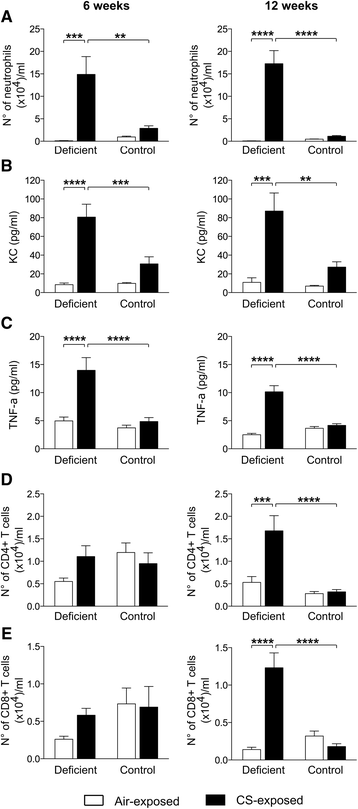
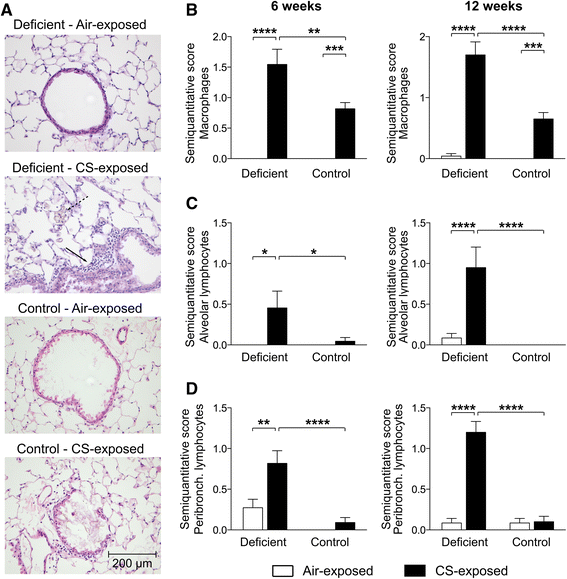
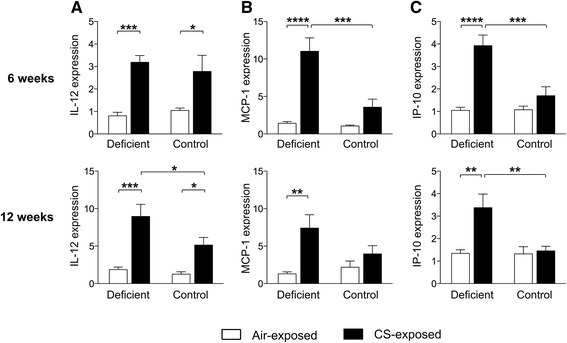
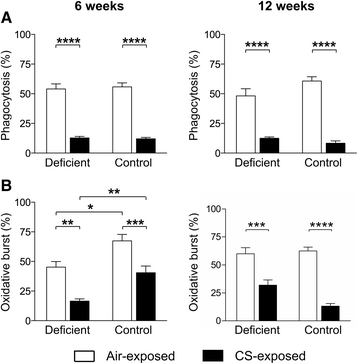
Results: Vitamin D deficient mice exhibited an accelerated lung function decline following CS exposure compared to control mice. Furthermore, early signs of emphysema were only observed in CS-exposed vitamin D deficient mice, which was accompanied by elevated levels of MMP-12 in the lung. Vitamin D deficient mice showed exacerbated infiltration of inflammatory cells in the airways and lung parenchyma after both subacute and chronic CS exposure compared to control mice. Furthermore, elevated levels of typical proinflammatory cytokines and chemokines could be detected in the bronchoalveolar lavage fluid (KC and TNF-α) and lung tissue (IP-10, MCP-1, IL-12) of CS-exposed vitamin D deficient mice compared to control mice. Finally, although CS greatly impaired the ex vivo phagocytic and oxidative burst function of alveolar macrophages, vitamin D deficient mice did not feature an additional defect.
Conclusions: Our data demonstrate that vitamin D deficiency both accelerates and aggravates the development of characteristic disease features of COPD. As vitamin D deficiency is highly prevalent, large randomized trials exploring effects of vitamin D supplementation on lung function decline and COPD onset are needed.
Figures
Similar articles
-
Time-course effects of aerobic physical training in the prevention of cigarette smoke-induced COPD.J Appl Physiol (1985). 2017 Sep 1;123(3):674-683. doi: 10.1152/japplphysiol.00819.2016. Epub 2017 Jul 20. J Appl Physiol (1985). 2017. PMID: 28729393
-
Cigarette smoke-induced pulmonary inflammation and emphysema are attenuated in CCR6-deficient mice.J Immunol. 2006 Oct 1;177(7):4350-9. doi: 10.4049/jimmunol.177.7.4350. J Immunol. 2006. PMID: 16982869
-
Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis.Am J Physiol Cell Physiol. 2018 Jan 1;314(1):C73-C87. doi: 10.1152/ajpcell.00110.2016. Epub 2016 Jul 13. Am J Physiol Cell Physiol. 2018. PMID: 27413169 Free PMC article.
-
Increased surfactant protein-D and foamy macrophages in smoking-induced mouse emphysema.Respirology. 2007 Mar;12(2):191-201. doi: 10.1111/j.1440-1843.2006.01009.x. Respirology. 2007. PMID: 17298450 Review.
-
Role of apoptosis in the pathogenesis of COPD and pulmonary emphysema.Respir Res. 2006 Mar 30;7(1):53. doi: 10.1186/1465-9921-7-53. Respir Res. 2006. PMID: 16571143 Free PMC article. Review.
Cited by
-
Can Vitamin D and L-Cysteine Co-Supplementation Reduce 25(OH)-Vitamin D Deficiency and the Mortality Associated with COVID-19 in African Americans?J Am Coll Nutr. 2020 Nov-Dec;39(8):694-699. doi: 10.1080/07315724.2020.1789518. Epub 2020 Jul 13. J Am Coll Nutr. 2020. PMID: 32659175 Free PMC article.
-
Lung Macrophage Phenotypes and Functional Responses: Role in the Pathogenesis of COPD.Int J Mol Sci. 2018 Feb 15;19(2):582. doi: 10.3390/ijms19020582. Int J Mol Sci. 2018. PMID: 29462886 Free PMC article. Review.
-
Effect of simvastatin on MMPs and TIMPs in cigarette smoke-induced rat COPD model.Int J Chron Obstruct Pulmon Dis. 2017 Feb 22;12:717-724. doi: 10.2147/COPD.S110520. eCollection 2017. Int J Chron Obstruct Pulmon Dis. 2017. PMID: 28260878 Free PMC article.
-
Vitamin D Actions: The Lung Is a Major Target for Vitamin D, FGF23, and Klotho.JBMR Plus. 2021 Nov 18;5(12):e10569. doi: 10.1002/jbm4.10569. eCollection 2021 Dec. JBMR Plus. 2021. PMID: 34950829 Free PMC article.
-
Cigarette smoke up-regulates PDE3 and PDE4 to decrease cAMP in airway cells.Br J Pharmacol. 2018 Jul;175(14):2988-3006. doi: 10.1111/bph.14347. Epub 2018 Jun 3. Br J Pharmacol. 2018. PMID: 29722436 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

