Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2)
- PMID: 26374899
- PMCID: PMC4646257
- DOI: 10.1074/jbc.M115.679043
Apolipoprotein E Is a Ligand for Triggering Receptor Expressed on Myeloid Cells 2 (TREM2)
Abstract
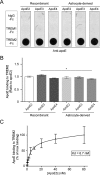
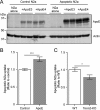
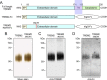
Several heterozygous missense mutations in the triggering receptor expressed on myeloid cells 2 (TREM2) have recently been linked to risk for a number of neurological disorders including Alzheimer disease (AD), Parkinson disease, and frontotemporal dementia. These discoveries have re-ignited interest in the role of neuroinflammation in the pathogenesis of neurodegenerative diseases. TREM2 is highly expressed in microglia, the resident immune cells of the central nervous system. Along with its adaptor protein, DAP12, TREM2 regulates inflammatory cytokine release and phagocytosis of apoptotic neurons. Here, we report apolipoprotein E (apoE) as a novel ligand for TREM2. Using a biochemical assay, we demonstrated high-affinity binding of apoE to human TREM2. The functional significance of this binding was highlighted by increased phagocytosis of apoE-bound apoptotic N2a cells by primary microglia in a manner that depends on TREM2 expression. Moreover, when the AD-associated TREM2-R47H mutant was used in biochemical assays, apoE binding was vastly reduced. Our data demonstrate that apoE-TREM2 interaction in microglia plays critical roles in modulating phagocytosis of apoE-bound apoptotic neurons and establish a critical link between two proteins whose genes are strongly linked to the risk for AD.
Keywords: Alzheimer disease; TREM2; apolipoprotein; apolipoprotein E (apoE); microglia; neuroinflammation.
© 2015 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures


Similar articles
-
The Role of APOE and TREM2 in Alzheimer's Disease-Current Understanding and Perspectives.Int J Mol Sci. 2018 Dec 26;20(1):81. doi: 10.3390/ijms20010081. Int J Mol Sci. 2018. PMID: 30587772 Free PMC article. Review.
-
The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases.Immunity. 2017 Sep 19;47(3):566-581.e9. doi: 10.1016/j.immuni.2017.08.008. Immunity. 2017. PMID: 28930663 Free PMC article.
-
Different mechanisms of apolipoprotein E isoform-dependent modulation of prostaglandin E2 production and triggering receptor expressed on myeloid cells 2 (TREM2) expression after innate immune activation of microglia.FASEB J. 2015 May;29(5):1754-62. doi: 10.1096/fj.14-262683. Epub 2015 Jan 15. FASEB J. 2015. PMID: 25593125 Free PMC article.
-
The Triggering Receptor Expressed on Myeloid Cells 2 Binds Apolipoprotein E.J Biol Chem. 2015 Oct 23;290(43):26033-42. doi: 10.1074/jbc.M115.677286. Epub 2015 Sep 15. J Biol Chem. 2015. PMID: 26374897 Free PMC article.
-
Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight.Nat Rev Immunol. 2018 Dec;18(12):759-772. doi: 10.1038/s41577-018-0051-1. Nat Rev Immunol. 2018. PMID: 30140051 Free PMC article. Review.
Cited by
-
Chronic TREM2 activation exacerbates Aβ-associated tau seeding and spreading.J Exp Med. 2023 Jan 2;220(1):e20220654. doi: 10.1084/jem.20220654. Epub 2022 Oct 11. J Exp Med. 2023. PMID: 36219197 Free PMC article.
-
TREM2/PLCγ2 signalling in immune cells: function, structural insight, and potential therapeutic modulation.Mol Neurodegener. 2021 Apr 6;16(1):22. doi: 10.1186/s13024-021-00436-5. Mol Neurodegener. 2021. PMID: 33823896 Free PMC article. Review.
-
TREM2 Haplodeficiency in Mice and Humans Impairs the Microglia Barrier Function Leading to Decreased Amyloid Compaction and Severe Axonal Dystrophy.Neuron. 2016 May 18;90(4):724-39. doi: 10.1016/j.neuron.2016.05.003. Neuron. 2016. PMID: 27196974 Free PMC article.
-
ApoE4 reduction: An emerging and promising therapeutic strategy for Alzheimer's disease.Neurobiol Aging. 2022 Jul;115:20-28. doi: 10.1016/j.neurobiolaging.2022.03.011. Epub 2022 Mar 22. Neurobiol Aging. 2022. PMID: 35453035 Free PMC article. Review.
-
Inflammation: the link between comorbidities, genetics, and Alzheimer's disease.J Neuroinflammation. 2018 Sep 24;15(1):276. doi: 10.1186/s12974-018-1313-3. J Neuroinflammation. 2018. PMID: 30249283 Free PMC article. Review.
References
-
- Jack C. R. Jr., Knopman D. S., Jagust W. J., Petersen R. C., Weiner M. W., Aisen P. S., Shaw L. M., Vemuri P., Wiste H. J., Weigand S. D., Lesnick T. G., Pankratz V. S., Donohue M. C., and Trojanowski J. Q. (2013) Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 - PMC - PubMed
-
- Paloneva J., Manninen T., Christman G., Hovanes K., Mandelin J., Adolfsson R., Bianchin M., Bird T., Miranda R., Salmaggi A., Tranebjaerg L., Konttinen Y., and Peltonen L. (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 - PMC - PubMed
-
- Kondo T., Takahashi K., Kohara N., Takahashi Y., Hayashi S., Takahashi H., Matsuo H., Yamazaki M., Inoue K., Miyamoto K., Yamamura T. (2002) Heterogeneity of presenile dementia with bone cysts (Nasu-Hakola disease): three genetic forms. Neurology 59, 1105–1107 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P50AG016574/AG/NIA NIH HHS/United States
- R01AG046205/AG/NIA NIH HHS/United States
- P01 NS074969/NS/NINDS NIH HHS/United States
- R01 AG027924/AG/NIA NIH HHS/United States
- R01AG027924/AG/NIA NIH HHS/United States
- R01 AG046205/AG/NIA NIH HHS/United States
- R01AG035355/AG/NIA NIH HHS/United States
- P50 AG016574/AG/NIA NIH HHS/United States
- RF1 AG051504/AG/NIA NIH HHS/United States
- P01 AG030128/AG/NIA NIH HHS/United States
- R01 AG035355/AG/NIA NIH HHS/United States
- P01AG30128/AG/NIA NIH HHS/United States
- P01NS074969/NS/NINDS NIH HHS/United States
- RF1 AG046205/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

