Integrative Model of Oxidative Stress Adaptation in the Fungal Pathogen Candida albicans
- PMID: 26368573
- PMCID: PMC4569071
- DOI: 10.1371/journal.pone.0137750
Integrative Model of Oxidative Stress Adaptation in the Fungal Pathogen Candida albicans
Abstract
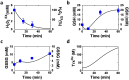
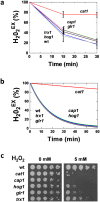
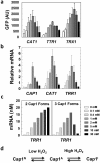
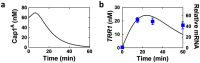
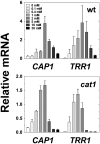
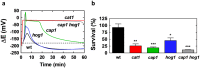
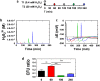
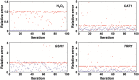
The major fungal pathogen of humans, Candida albicans, mounts robust responses to oxidative stress that are critical for its virulence. These responses counteract the reactive oxygen species (ROS) that are generated by host immune cells in an attempt to kill the invading fungus. Knowledge of the dynamical processes that instigate C. albicans oxidative stress responses is required for a proper understanding of fungus-host interactions. Therefore, we have adopted an interdisciplinary approach to explore the dynamical responses of C. albicans to hydrogen peroxide (H2O2). Our deterministic mathematical model integrates two major oxidative stress signalling pathways (Cap1 and Hog1 pathways) with the three major antioxidant systems (catalase, glutathione and thioredoxin systems) and the pentose phosphate pathway, which provides reducing equivalents required for oxidative stress adaptation. The model encapsulates existing knowledge of these systems with new genomic, proteomic, transcriptomic, molecular and cellular datasets. Our integrative approach predicts the existence of alternative states for the key regulators Cap1 and Hog1, thereby suggesting novel regulatory behaviours during oxidative stress. The model reproduces both existing and new experimental observations under a variety of scenarios. Time- and dose-dependent predictions of the oxidative stress responses for both wild type and mutant cells have highlighted the different temporal contributions of the various antioxidant systems during oxidative stress adaptation, indicating that catalase plays a critical role immediately following stress imposition. This is the first model to encapsulate the dynamics of the transcriptional response alongside the redox kinetics of the major antioxidant systems during H2O2 stress in C. albicans.
Conflict of interest statement
Figures

Similar articles
-
Adaptive tolerance to oxidative stress and the induction of antioxidant enzymatic activities in Candida albicans are independent of the Hog1 and Cap1-mediated pathways.FEMS Yeast Res. 2010 Sep;10(6):747-56. doi: 10.1111/j.1567-1364.2010.00654.x. Epub 2010 Jun 7. FEMS Yeast Res. 2010. PMID: 20608985
-
Farnesol induces hydrogen peroxide resistance in Candida albicans yeast by inhibiting the Ras-cyclic AMP signaling pathway.Eukaryot Cell. 2010 Apr;9(4):569-77. doi: 10.1128/EC.00321-09. Epub 2010 Jan 29. Eukaryot Cell. 2010. PMID: 20118211 Free PMC article.
-
Systematic identification and characterization of five transcription factors mediating the oxidative stress response in Candida albicans.Microb Pathog. 2024 Feb;187:106507. doi: 10.1016/j.micpath.2023.106507. Epub 2023 Dec 23. Microb Pathog. 2024. PMID: 38145792
-
Oxidative stress responses in the human fungal pathogen, Candida albicans.Biomolecules. 2015 Feb 25;5(1):142-65. doi: 10.3390/biom5010142. Biomolecules. 2015. PMID: 25723552 Free PMC article. Review.
-
Epigenetic Control of Oxidative Stresses by Histone Acetyltransferases in Candida albicans.J Microbiol Biotechnol. 2018 Feb 28;28(2):181-189. doi: 10.4014/jmb.1707.07029. J Microbiol Biotechnol. 2018. PMID: 29169224 Review.
Cited by
-
On the Anti-Cancer Effect of Cold Atmospheric Plasma and the Possible Role of Catalase-Dependent Apoptotic Pathways.Cells. 2020 Oct 21;9(10):2330. doi: 10.3390/cells9102330. Cells. 2020. PMID: 33096638 Free PMC article.
-
Candida albicans FRE8 encodes a member of the NADPH oxidase family that produces a burst of ROS during fungal morphogenesis.PLoS Pathog. 2017 Dec 1;13(12):e1006763. doi: 10.1371/journal.ppat.1006763. eCollection 2017 Dec. PLoS Pathog. 2017. PMID: 29194441 Free PMC article.
-
Candida albicans pathways that protect against organic peroxides and lipid peroxidation.PLoS Genet. 2024 Oct 21;20(10):e1011455. doi: 10.1371/journal.pgen.1011455. eCollection 2024 Oct. PLoS Genet. 2024. PMID: 39432552 Free PMC article.
-
Elevated catalase expression in a fungal pathogen is a double-edged sword of iron.PLoS Pathog. 2017 May 22;13(5):e1006405. doi: 10.1371/journal.ppat.1006405. eCollection 2017 May. PLoS Pathog. 2017. PMID: 28542620 Free PMC article.
-
Extending the Proteomic Characterization of Candida albicans Exposed to Stress and Apoptotic Inducers through Data-Independent Acquisition Mass Spectrometry.mSystems. 2021 Oct 26;6(5):e0094621. doi: 10.1128/mSystems.00946-21. Epub 2021 Oct 5. mSystems. 2021. PMID: 34609160 Free PMC article.
References
-
- Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Transl Med. 2012;4(165):165rv13 . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 097377/WT_/Wellcome Trust/United Kingdom
- BB/K016393/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F005210/1-2/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/K017365/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/F00513X/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

