IP3R deficit underlies loss of salivary fluid secretion in Sjögren's Syndrome
- PMID: 26365984
- PMCID: PMC4568516
- DOI: 10.1038/srep13953
IP3R deficit underlies loss of salivary fluid secretion in Sjögren's Syndrome
Abstract
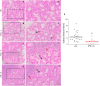
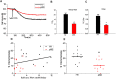
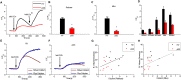
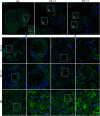
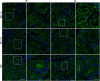
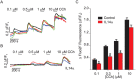
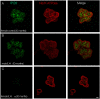
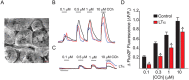
The autoimmune exocrinopathy, Sjögren's syndrome (SS), is associated with secretory defects in patients, including individuals with mild lymphocytic infiltration and minimal glandular damage. The mechanism(s) underlying the secretory dysfunction is not known. We have used minor salivary gland biopsies from SS patients and healthy individuals to assess acinar cell function in morphologically intact glandular areas. We report that agonist-regulated intracellular Ca(2+) release, critically required for Ca(2+) entry and fluid secretion, is defective in acini from SS patients. Importantly, these acini displayed reduction in IP3R2 and IP3R3, but not AQP5 or STIM1. Similar decreases in IP3R and carbachol (CCh)-stimulated [Ca(2+)]i elevation were detected in acinar cells from lymphotoxin-alpha (LTα) transgenic (TG) mice, a model for (SS). Treatment of salivary glands from healthy individuals with LT α, a cytokine linked to disease progression in SS and IL14α mice, reduced Ca(2+) signaling. Together, our findings reveal novel IP3R deficits in acinar cells that underlie secretory dysfunction in SS patients.
Figures
Similar articles
-
Synaptotagmin-1 overexpression under inflammatory conditions affects secretion in salivary glands from Sjögren's syndrome patients.J Autoimmun. 2019 Feb;97:88-99. doi: 10.1016/j.jaut.2018.10.019. Epub 2018 Oct 31. J Autoimmun. 2019. PMID: 30391023
-
Restoration of CFTR Activity in Ducts Rescues Acinar Cell Function and Reduces Inflammation in Pancreatic and Salivary Glands of Mice.Gastroenterology. 2017 Oct;153(4):1148-1159. doi: 10.1053/j.gastro.2017.06.011. Epub 2017 Jun 19. Gastroenterology. 2017. PMID: 28634110 Free PMC article.
-
A role for lymphotoxin in primary Sjogren's disease.J Immunol. 2010 Nov 15;185(10):6355-63. doi: 10.4049/jimmunol.1001520. Epub 2010 Oct 15. J Immunol. 2010. PMID: 20952683
-
Calcium signaling defects underlying salivary gland dysfunction.Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1771-1777. doi: 10.1016/j.bbamcr.2018.07.002. Epub 2018 Jul 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30006140 Review.
-
Laminin isoform profiles in salivary glands in Sjögren's syndrome.Adv Clin Chem. 2011;55:35-59. doi: 10.1016/b978-0-12-387042-1.00003-4. Adv Clin Chem. 2011. PMID: 22126023 Review.
Cited by
-
Dysregulated Ca2+ signaling, fluid secretion, and mitochondrial function in a mouse model of early Sjögren's disease.Elife. 2024 Sep 11;13:RP97069. doi: 10.7554/eLife.97069. Elife. 2024. PMID: 39259200 Free PMC article.
-
Epithelial-immune cell interplay in primary Sjögren syndrome salivary gland pathogenesis.Nat Rev Rheumatol. 2021 Jun;17(6):333-348. doi: 10.1038/s41584-021-00605-2. Epub 2021 Apr 28. Nat Rev Rheumatol. 2021. PMID: 33911236 Free PMC article. Review.
-
Transcriptomic Profile of Genes Encoding Proteins Involved in Pathogenesis of Sjögren's Syndrome Related Xerostomia-Molecular and Clinical Trial.J Clin Med. 2020 Oct 14;9(10):3299. doi: 10.3390/jcm9103299. J Clin Med. 2020. PMID: 33066537 Free PMC article.
-
Aquaporins in Glandular Secretion.Adv Exp Med Biol. 2023;1398:225-249. doi: 10.1007/978-981-19-7415-1_16. Adv Exp Med Biol. 2023. PMID: 36717498
-
IL-17 drives salivary gland dysfunction via inhibiting TRPC1-mediated calcium movement in Sjögren's syndrome.Clin Transl Immunology. 2021 Apr 29;10(4):e1277. doi: 10.1002/cti2.1277. eCollection 2021. Clin Transl Immunology. 2021. PMID: 33968407 Free PMC article.
References
-
- Hansen A., Lipsky P. E. & Dorner T. Immunopathogenesis of primary Sjogren’s syndrome: implications for disease management and therapy. Current opinion in rheumatology 17, 558–565 (2005). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

