G2/M Cell Cycle Arrest and Tumor Selective Apoptosis of Acute Leukemia Cells by a Promising Benzophenone Thiosemicarbazone Compound
- PMID: 26360247
- PMCID: PMC4567328
- DOI: 10.1371/journal.pone.0136878
G2/M Cell Cycle Arrest and Tumor Selective Apoptosis of Acute Leukemia Cells by a Promising Benzophenone Thiosemicarbazone Compound
Abstract
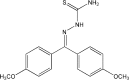
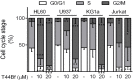
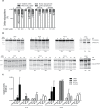
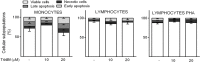
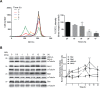
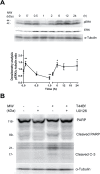
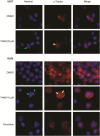
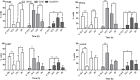
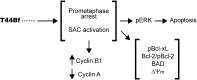
Anti-mitotic therapies have been considered a hallmark in strategies against abnormally proliferating cells. Focusing on the extensively studied family of thiosemicarbazone (TSC) compounds, we have previously identified 4,4'-dimethoxybenzophenone thiosemicarbazone (T44Bf) as a promising pharmacological compound in a panel of human leukemia cell lines (HL60, U937, KG1a and Jurkat). Present findings indicate that T44Bf-mediated antiproliferative effects are associated with a reversible chronic mitotic arrest caused by defects in chromosome alignment, followed by induced programmed cell death. Furthermore, T44Bf selectively induces apoptosis in leukemia cell lines when compared to normal peripheral blood mononuclear cells. The underlying mechanism of action involves the activation of the mitochondria signaling pathway, with loss of mitochondrial membrane potential and sustained phosphorylation of anti-apoptotic protein Bcl-xL as well as increased Bcl-2 (enhanced phosphorylated fraction) and pro-apoptotic protein Bad levels. In addition, ERK signaling pathway activation was found to be a requisite for T44Bf apoptotic activity. Our findings further describe a novel activity for a benzophenone thiosemicarbazone and propose T44Bf as a promising anti-mitotic prototype to develop chemotherapeutic agents to treat acute leukemia malignancies.
Conflict of interest statement
Figures
Similar articles
-
N-benzyl-N-methyldecan-1-amine, a phenylamine derivative isolated from garlic cloves, induces G2/M phase arrest and apoptosis in U937 human leukemia cells.Oncol Rep. 2014 Jul;32(1):373-81. doi: 10.3892/or.2014.3215. Epub 2014 May 23. Oncol Rep. 2014. PMID: 24859825
-
Inhibition of JNK2 and JNK3 by JNK inhibitor IX induces prometaphase arrest-dependent apoptotic cell death in human Jurkat T cells.Biochem Biophys Res Commun. 2014 Sep 26;452(3):845-51. doi: 10.1016/j.bbrc.2014.09.015. Epub 2014 Sep 16. Biochem Biophys Res Commun. 2014. PMID: 25218503
-
Kaempferol Activates G₂-Checkpoint of the Cell Cycle Resulting in G₂-Arrest and Mitochondria-Dependent Apoptosis in Human Acute Leukemia Jurkat T Cells.J Microbiol Biotechnol. 2016 Feb;26(2):287-94. doi: 10.4014/jmb.1511.11054. J Microbiol Biotechnol. 2016. PMID: 26699757
-
Mitochondria and Acute Leukemia: A Clinician's Perspective.Int J Mol Sci. 2024 Sep 7;25(17):9704. doi: 10.3390/ijms25179704. Int J Mol Sci. 2024. PMID: 39273651 Free PMC article. Review.
-
Targeting the Mitotic Catastrophe Signaling Pathway in Cancer.Mediators Inflamm. 2015;2015:146282. doi: 10.1155/2015/146282. Epub 2015 Sep 27. Mediators Inflamm. 2015. PMID: 26491220 Free PMC article. Review.
Cited by
-
The role of oxidative stress in activity of anticancer thiosemicarbazones.Oncotarget. 2018 Apr 3;9(25):17689-17710. doi: 10.18632/oncotarget.24844. eCollection 2018 Apr 3. Oncotarget. 2018. PMID: 29707141 Free PMC article.
-
Crystal Protein of a Novel Bacillus thuringiensis Strain Inducing Cell Cycle Arrest and Apoptotic Cell Death in Human Leukemic Cells.Sci Rep. 2019 Jul 4;9(1):9661. doi: 10.1038/s41598-019-45928-z. Sci Rep. 2019. PMID: 31273223 Free PMC article.
-
Inhibition of growth of Asian keloid cells with human umbilical cord Wharton's jelly stem cell-conditioned medium.Stem Cell Res Ther. 2020 Feb 21;11(1):78. doi: 10.1186/s13287-020-01609-7. Stem Cell Res Ther. 2020. PMID: 32085797 Free PMC article.
-
Novel pyridinecarboxaldehyde thiosemicarbazone conjugated magnetite nanoparticulates (MNPs) promote apoptosis in human lung cancer A549 cells.J Biol Inorg Chem. 2020 Feb;25(1):13-22. doi: 10.1007/s00775-019-01728-4. Epub 2019 Oct 19. J Biol Inorg Chem. 2020. PMID: 31630253
-
Synthesis of Carvacrol Derivatives as Potential New Anticancer Agent against Lung Cancer.Molecules. 2022 Jul 19;27(14):4597. doi: 10.3390/molecules27144597. Molecules. 2022. PMID: 35889476 Free PMC article.
References
-
- McCulloch EA. Stem cells in normal and leukemic hemopoiesis (Henry Stratton Lecture, 1982). Blood. 1983;62(1):1–13. - PubMed
-
- Bonnet D, Dick J. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature medicine. 1997;3(7):730–7. - PubMed
-
- Schimmer A, Hedley D, Penn L, Minden M. Receptor- and mitochondrial-mediated apoptosis in acute leukemia: a translational view. Blood. 2001;98(13):3541–53. - PubMed
-
- Tallman M, Gilliland D, Rowe J. Drug therapy for acute myeloid leukemia. Blood. 2005;106(4):1154–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

