Regulation of PACT-Mediated Protein Kinase Activation by the OV20.0 Protein of Orf Virus
- PMID: 26355092
- PMCID: PMC4645672
- DOI: 10.1128/JVI.01739-15
Regulation of PACT-Mediated Protein Kinase Activation by the OV20.0 Protein of Orf Virus
Abstract
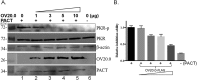
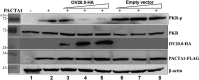
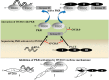
Double-stranded RNA (dsRNA)-activated protein kinase (PKR), a major component of the cellular antiviral system, is activated by the binding of either dsRNA or the cellular PKR activator, the PACT protein. The suppression of PKR activation is one of the main strategies that viruses employ to circumvent interferon signaling. Orf virus (ORFV), a parapoxvirus from the Poxviridae family, causes contagious pustular dermatitis in small ruminants. Previous studies have demonstrated that various OV20.0 isoforms, encoded by the OV20.0L gene, are able to inhibit PKR activation both by sequestering dsRNA and by physically interacting with PKR in vitro. Thus, this gene acts as a virulence factor of ORFV when tested using a mouse infection model. In the present study, the regions within OV20.0 that interact with dsRNA and with PKR have been mapped. Furthermore, this study demonstrates for the first time that OV20.0 is also able to interact with the dsRNA binding domain of PACT and that the presence of dsRNA strengthened the interaction of these two molecules. The presence of OV20.0 diminishes PKR phosphorylation when this is stimulated by PACT. Nevertheless, the association of OV20.0 with PKR, rather than with PACT, was found to be essential for reducing PACT-mediated PKR phosphorylation. These observations elucidate a new strategy whereby innate immunity can be evaded by ORFV.IMPORTANCE Our previous study indicated that ORFV's two OV20.0 isoforms act as a PKR antagonist via sequestering the PKR activator, dsRNA, and by interacting with PKR, leading to an inhibition of PKR activation (Y. Y. Tseng, F. Y. Lin, S. F. Cheng, D. Tscharke, S. Chulakasian, C. C. Chou, Y. F. Liu, W. S. Chang, M. L. Wong, and W. L. Hsu, J Virol 89:4966-4979, 2015, doi:10.1128/JVI.03714-14). In the current study, the possible mechanisms by which OV20.0 protein counteracts PKR activation were studied in depth. OV20.0 is able to bind PKR and its two activators, dsRNA and PACT. In addition, OV20.0 binds directly to the RNA binding domains (RBDs) of PKR, and this interaction does not require dsRNA. Moreover, OV20.0 interacts with or occupies the RBD2 and the kinase domain of PKR, which then prevents PACT binding to PKR. Finally, OV20.0 associates with PACT via the RBDs, which may reduce the ability of PACT to induce PKR activation. The findings in this study provide new concepts in relation to how ORFV modulates PKR activation.
Copyright © 2015, American Society for Microbiology. All Rights Reserved.
Figures






Similar articles
-
Adenosine Deaminase Acting on RNA 1 Associates with Orf Virus OV20.0 and Enhances Viral Replication.J Virol. 2019 Mar 21;93(7):e01912-18. doi: 10.1128/JVI.01912-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651363 Free PMC article.
-
K160 in the RNA-binding domain of the orf virus virulence factor OV20.0 is critical for its functions in counteracting host antiviral defense.FEBS Lett. 2021 Jun;595(12):1721-1733. doi: 10.1002/1873-3468.14099. Epub 2021 May 27. FEBS Lett. 2021. PMID: 33909294
-
Functional analysis of the short isoform of orf virus protein OV20.0.J Virol. 2015 May;89(9):4966-79. doi: 10.1128/JVI.03714-14. Epub 2015 Feb 18. J Virol. 2015. PMID: 25694596 Free PMC article.
-
PACT and PKR: turning on NF-kappa B in the absence of virus.Sci STKE. 2001 Jul 3;2001(89):re1. doi: 10.1126/stke.2001.89.re1. Sci STKE. 2001. PMID: 11752660 Review.
-
Activation of PKR: an open and shut case?Trends Biochem Sci. 2007 Feb;32(2):57-62. doi: 10.1016/j.tibs.2006.12.003. Epub 2006 Dec 29. Trends Biochem Sci. 2007. PMID: 17196820 Free PMC article. Review.
Cited by
-
Adenosine Deaminase Acting on RNA 1 Associates with Orf Virus OV20.0 and Enhances Viral Replication.J Virol. 2019 Mar 21;93(7):e01912-18. doi: 10.1128/JVI.01912-18. Print 2019 Apr 1. J Virol. 2019. PMID: 30651363 Free PMC article.
-
Virus-Host Protein Interaction Network of the Hepatitis E Virus ORF2-4 by Mammalian Two-Hybrid Assays.Viruses. 2023 Dec 12;15(12):2412. doi: 10.3390/v15122412. Viruses. 2023. PMID: 38140653 Free PMC article.
-
A tale of two proteins: PACT and PKR and their roles in inflammation.FEBS J. 2021 Nov;288(22):6365-6391. doi: 10.1111/febs.15691. Epub 2021 Jan 15. FEBS J. 2021. PMID: 33387379 Free PMC article. Review.
-
Inhibition of PKR by Viruses.Front Microbiol. 2021 Oct 25;12:757238. doi: 10.3389/fmicb.2021.757238. eCollection 2021. Front Microbiol. 2021. PMID: 34759908 Free PMC article. Review.
-
ADAR1 and PACT contribute to efficient translation of transcripts containing HIV-1 trans-activating response (TAR) element.Biochem J. 2017 Mar 23;474(7):1241-1257. doi: 10.1042/BCJ20160964. Biochem J. 2017. PMID: 28167698 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

