Serum MicroRNAs as Potential Biomarkers for Early Diagnosis of Hepatitis C Virus-Related Hepatocellular Carcinoma in Egyptian Patients
- PMID: 26352740
- PMCID: PMC4564244
- DOI: 10.1371/journal.pone.0137706
Serum MicroRNAs as Potential Biomarkers for Early Diagnosis of Hepatitis C Virus-Related Hepatocellular Carcinoma in Egyptian Patients
Abstract
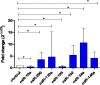
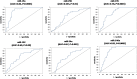
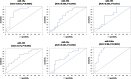
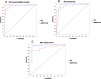
Circulating microRNAs are deregulated in liver fibrosis and hepatocellular carcinoma (HCC) and are candidate biomarkers. This study investigated the potential of serum microRNAs; miR-19a, miR-296, miR-130a, miR-195, miR-192, miR-34a, and miR-146a as early diagnostic biomarkers for hepatitis C virus (HCV)-related HCC. As how these microRNAs change during liver fibrosis progression is not clear, we explored their serum levels during fibrosis progression in HCV-associated chronic liver disease (CLD) and if they could serve as non-invasive biomarkers for fibrosis progression to HCC. 112 Egyptian HCV-HCC patients, 125 non-malignant HCV-CLD patients, and 42 healthy controls were included. CLD patients were subdivided according to Metavir fibrosis-scoring. Serum microRNAs were measured by qRT-PCR custom array. Serum microRNAs were deregulated in HCC versus controls, and except miR-130a, they were differentially expressed between HCC and CLD or late fibrosis (F3-F4) subgroup. Serum microRNAs were not significantly different between individual fibrosis-stages or between F1-F2 (early/moderate fibrosis) and F3-F4. Only miR-19a was significantly downregulated from liver fibrosis (F1-F3) to cirrhosis (F4) to HCC. Individual microRNAs discriminated HCC from controls, and except miR-130a, they distinguished HCC from CLD or F3-F4 patients by receiver-operating-characteristic analysis. Multivariate logistic analysis revealed a panel of four microRNAs (miR-19a, miR-195, miR-192, and miR-146a) with high diagnostic accuracy for HCC (AUC = 0.946). The microRNA panel also discriminated HCC from controls (AUC = 0.949), CLD (AUC = 0.945), and F3-F4 (AUC = 0.955). Studied microRNAs were positively correlated in HCC group. miR-19a and miR-34a were correlated with portal vein thrombosis and HCC staging scores, respectively. In conclusion, studied microRNAs, but not miR-130a, could serve as potential early biomarkers for HCC in high-risk groups, with miR-19a as a biomarker for liver fibrosis progression to cirrhosis to HCC. We identified a panel of four serum microRNAs with high accuracy in HCC diagnosis. Additional studies are required to confirm this panel and test its prognostic significance.
Conflict of interest statement
Figures




Similar articles
-
Serum microRNAs as predictors for liver fibrosis staging in hepatitis C virus-associated chronic liver disease patients.J Viral Hepat. 2017 Aug;24(8):636-644. doi: 10.1111/jvh.12696. Epub 2017 Mar 13. J Viral Hepat. 2017. PMID: 28211229
-
Evaluation of miR-331-3p and miR-23b-3p as serum biomarkers for hepatitis c virus-related hepatocellular carcinoma at early stage.Clin Res Hepatol Gastroenterol. 2020 Feb;44(1):21-28. doi: 10.1016/j.clinre.2019.03.011. Epub 2019 Apr 30. Clin Res Hepatol Gastroenterol. 2020. PMID: 31053500
-
Validation of a serum microRNA panel as biomarkers for early diagnosis of hepatocellular carcinoma post-hepatitis C infection in Egyptian patients.World J Gastroenterol. 2017 Jun 7;23(21):3864-3875. doi: 10.3748/wjg.v23.i21.3864. World J Gastroenterol. 2017. PMID: 28638226 Free PMC article.
-
Identification of circulating MicroRNAs as novel potential biomarkers for hepatocellular carcinoma detection: a systematic review and meta-analysis.Clin Transl Oncol. 2015 Sep;17(9):684-93. doi: 10.1007/s12094-015-1294-y. Epub 2015 May 9. Clin Transl Oncol. 2015. PMID: 25956842 Review.
-
MicroRNAs as Biomarkers for Liver Disease and Hepatocellular Carcinoma.Int J Mol Sci. 2016 Feb 24;17(3):280. doi: 10.3390/ijms17030280. Int J Mol Sci. 2016. PMID: 26927063 Free PMC article. Review.
Cited by
-
The Current Status of the Liver Liquid Biopsy in MASH Related HCC: Overview and Future Directions.Biomolecules. 2023 Sep 9;13(9):1369. doi: 10.3390/biom13091369. Biomolecules. 2023. PMID: 37759769 Free PMC article. Review.
-
Extracellular vesicles as a new hope for diagnosis and therapeutic intervention for hepatocellular carcinoma.Cancer Med. 2021 Dec;10(23):8253-8271. doi: 10.1002/cam4.4370. Epub 2021 Oct 28. Cancer Med. 2021. PMID: 34708589 Free PMC article. Review.
-
A meta-analysis of the diagnostic value of microRNA-1246 for malignant tumors.Medicine (Baltimore). 2019 May;98(22):e15848. doi: 10.1097/MD.0000000000015848. Medicine (Baltimore). 2019. PMID: 31145333 Free PMC article.
-
Eag1 channels as potential early-stage biomarkers of hepatocellular carcinoma.Biologics. 2016 Sep 20;10:139-148. doi: 10.2147/BTT.S87402. eCollection 2016. Biologics. 2016. PMID: 27703327 Free PMC article. Review.
-
Clinical significances and diagnostic utilities of both miR-215 and squamous cell carcinoma antigen-IgM versus alpha-fetoprotein in Egyptian patients with hepatitis C virus-induced hepatocellular carcinoma.Clin Exp Gastroenterol. 2019 Feb 1;12:51-66. doi: 10.2147/CEG.S179832. eCollection 2019. Clin Exp Gastroenterol. 2019. PMID: 30774409 Free PMC article.
References
-
- Freedman LS, Edwards BK, Ries LAG, Young JL, editors (2006) Cancer Incidence in Four Member Countries (Cyprus, Egypt, Israel, and Jordan) of the Middle East Cancer Consortium (MECC) Compared with US SEER. Bethesda, MD: NIH Pub: National Cancer Institute.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

