In vivo capture and label-free detection of early metastatic cells
- PMID: 26348915
- PMCID: PMC4563812
- DOI: 10.1038/ncomms9094
In vivo capture and label-free detection of early metastatic cells
Abstract
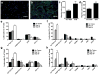
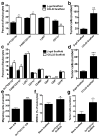
Breast cancer is a leading cause of death for women, with mortality resulting from metastasis. Metastases are often detected once tumour cells affect the function of solid organs, with a high disease burden limiting effective treatment. Here we report a method for the early detection of metastasis using an implanted scaffold to recruit and capture metastatic cells in vivo, which achieves high cell densities and reduces the tumour burden within solid organs 10-fold. Recruitment is associated with infiltration of immune cells, which include Gr1(hi)CD11b(+) cells. We identify metastatic cells in the scaffold through a label-free detection system using inverse spectroscopic optical coherence tomography, which identifies changes to nanoscale tissue architecture associated with the presence of tumour cells. For patients at risk of recurrence, scaffold implantation following completion of primary therapy has the potential to identify metastatic disease at the earliest stage, enabling initiation of therapy while the disease burden is low.
Conflict of interest statement
L.D.S. is an occasional consultant for Pioneer Biosolutions, which licenses some of the technology developed in his lab and used in this research. The remaining authors declare no competing financial interests.
Figures


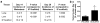

Similar articles
-
Enhanced Survival with Implantable Scaffolds That Capture Metastatic Breast Cancer Cells In Vivo.Cancer Res. 2016 Sep 15;76(18):5209-18. doi: 10.1158/0008-5472.CAN-15-2106. Cancer Res. 2016. PMID: 27635043 Free PMC article.
-
Hematogenic Dissemination of Triple-negative Versus Hormonal Receptor-positive Breast Cancer Cells.In Vivo. 2015 Jul-Aug;29(4):435-44. In Vivo. 2015. PMID: 26130788
-
Granulocytic immune infiltrates are essential for the efficient formation of breast cancer liver metastases.Breast Cancer Res. 2015 Mar 27;17(1):45. doi: 10.1186/s13058-015-0558-3. Breast Cancer Res. 2015. PMID: 25882816 Free PMC article.
-
Metastatic Cells Colonize Implantable Scaffold in Mice.J Natl Cancer Inst. 2015 Dec 27;108(1):djv421. doi: 10.1093/jnci/djv421. Print 2016 Jan. J Natl Cancer Inst. 2015. PMID: 26711666 No abstract available.
-
Circulating tumour cells, their role in metastasis and their clinical utility in lung cancer.Lung Cancer. 2012 Apr;76(1):19-25. doi: 10.1016/j.lungcan.2011.10.018. Epub 2011 Dec 29. Lung Cancer. 2012. PMID: 22209049 Review.
Cited by
-
Engineering Immune Tolerance with Biomaterials.Adv Healthc Mater. 2019 Feb;8(4):e1801419. doi: 10.1002/adhm.201801419. Epub 2019 Jan 3. Adv Healthc Mater. 2019. PMID: 30605264 Free PMC article. Review.
-
Circulating tumor cells can predict the prognosis of patients with non-small cell lung cancer after resection: a retrospective study.Transl Lung Cancer Res. 2021 Feb;10(2):995-1006. doi: 10.21037/tlcr-21-149. Transl Lung Cancer Res. 2021. PMID: 33718038 Free PMC article.
-
Multiple valence states of Fe boosting SERS activity of Fe3O4 nanoparticles and enabling effective SERS-MRI bimodal cancer imaging.Fundam Res. 2022 May 3;4(4):858-867. doi: 10.1016/j.fmre.2022.04.018. eCollection 2024 Jul. Fundam Res. 2022. PMID: 39156566 Free PMC article.
-
Biomaterials as Tools to Decode Immunity.Adv Mater. 2020 Apr;32(13):e1903367. doi: 10.1002/adma.201903367. Epub 2019 Nov 29. Adv Mater. 2020. PMID: 31782844 Free PMC article. Review.
-
Metastatic Conditioning of Myeloid Cells at a Subcutaneous Synthetic Niche Reflects Disease Progression and Predicts Therapeutic Outcomes.Cancer Res. 2020 Feb 1;80(3):602-612. doi: 10.1158/0008-5472.CAN-19-1932. Epub 2019 Oct 29. Cancer Res. 2020. PMID: 31662327 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

